40 reaction coordinate diagram organic chemistry
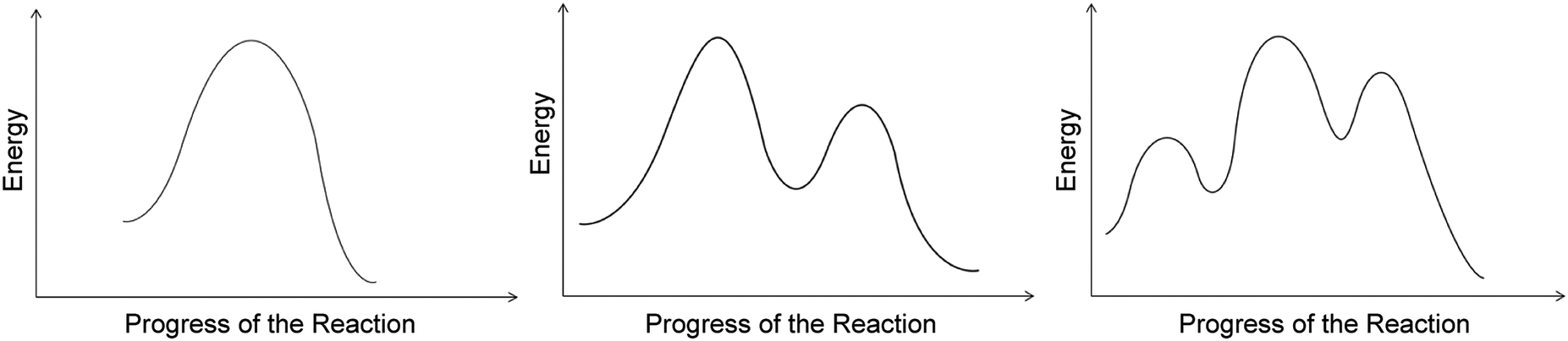
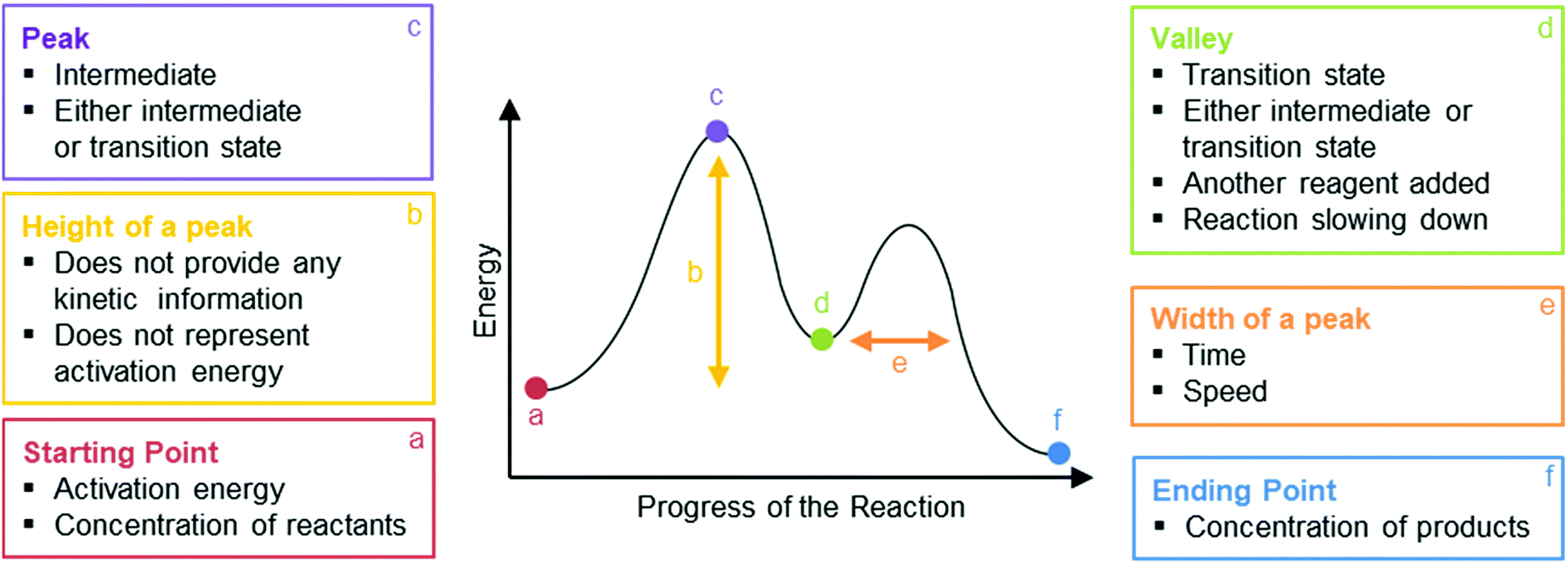
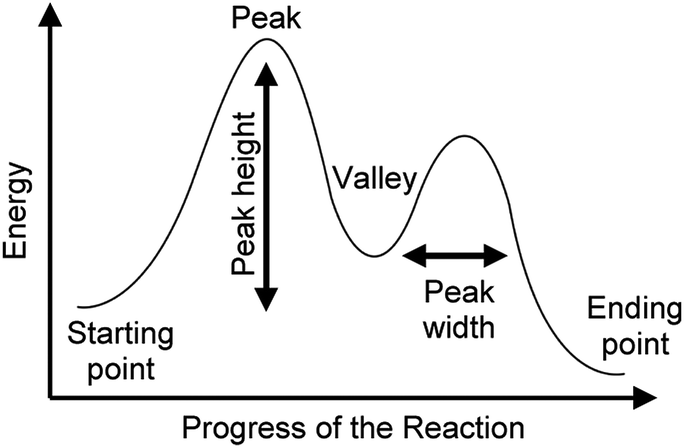
Predicting Acidity of Organic Compounds . Reaction Coordinate Diagrams and Reaction Rate . Conformations of Cyclohexane . Stereochemistry Part 1. Stereochemistry Part 2. Stereochemistry Part 3. Stereochemistry Part 4 Type 2 is to count the number of intermediates, transitions states, or the mechanistic steps in the reaction based on the diagram. The reaction diagram above has 2 intermediates and 3 transition states, so it is a 3-step reaction. Finally, the last question you can expect is a question about the shape or a nature of the transition state itself.
To get free access to the Interactive Organic course, just register with your email. No strings attached. No spam.A refreshing approach to organic chemistry!Welcome to the Interactive Organic chemistry course! We hope you find this course useful in your studies!This course covers all of the usual topics found in a two-semester, sophomore organic chemistry course.

Reaction coordinate diagram organic chemistry
energy diagram (or reaction energy diagram): a graph of the energy of a reaction against the progress of the reaction. enthalpy: a thermodynamic state function, generally measured in kilojoules per mole. In chemical reactions the enthalpy change (deltaH) is related to changes in the free energy (deltaG) and entropy (deltaS) by the equation: Illustrated Glossary of Organic Chemistry Rate determining step (rds; rate limiting step): The mechanism step with the greatest activation energy (i.e., the slowest step) and therefore the step that has the greatest influence on reaction rate . Organic chemistry students struggle with understanding the energetics of chemical reactions. Reaction coordinate diagrams are one tool that is widely used in organic chemistry classrooms to assist students with visualizing and explaining the energy changes that take place throughout a reaction.
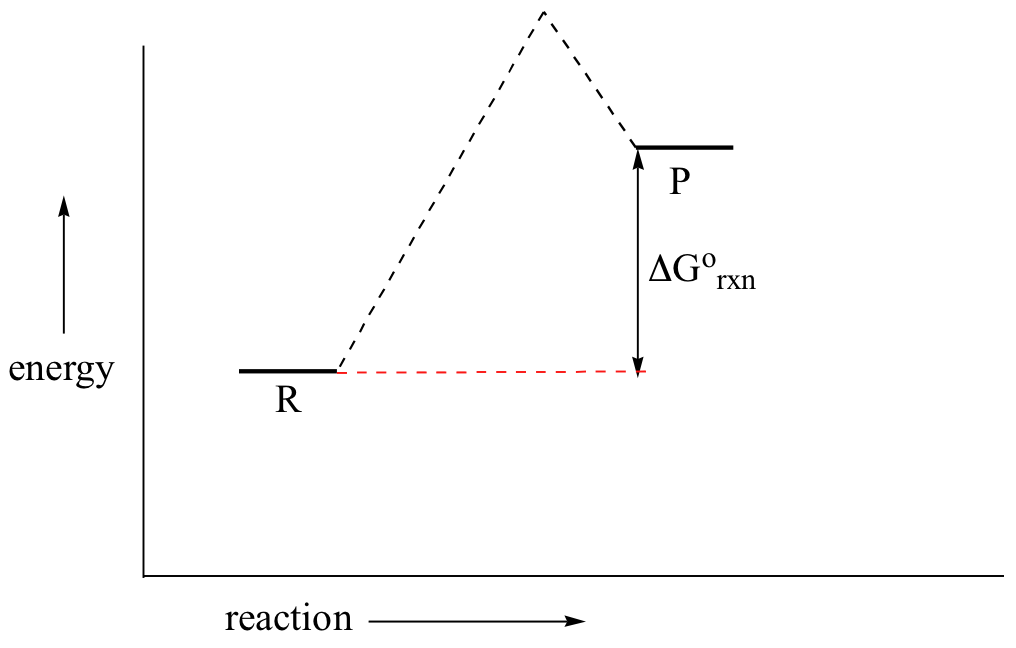
Reaction coordinate diagram organic chemistry. The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants. The formation or removal of carbon-carbon double bonds is also used in synthetic organic chemistry reactions to create desired organic molecules. One of these types of reactions is called a hydrogenation reaction, where a molecule of hydrogen (H 2) is added across a C-C double bond, reducing it to a C-C single bond. If this is done using ... The free energy diagrams for these reactions are as follows: The transition state is closer to the products when H20 is the nucleophile. The energy barrier is smaller, so the reaction is faster, and H2S is the stronger nucleophile. 1 H20 H2S H 20 Reaction coordinate Both reactions are endergonic because two new charges are produced. Both sets of Reaction coordinate diagrams | Organic Chemistry 1: An open textbook. 5.3. Reaction coordinate diagrams. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ...
PAPER : 5 ,Organic Chemistry- II (Reaction Mechanism-1) :4 , Intermediates, Transition states, Potential energy diagrams and Hammond's postulate Fig.7: Reaction coordinate diagram showing TS There may be more than one transition state for a reaction, generally for multi-step reactions. The In organic chemistry parlance, this is what is meant by "identify the symmetry elements that are preserved throughout the reaction". In organic chemistry, it is "conventional to be less formal with the notation" (Atkins's words; Molecular Quantum Mechanics 4ed, p 400), and so the irrep symbols are replaced with a series of S's (for a character ... A complete, free organic chemistry course with a free textbook online, free lecture videos, organic practice problems and multiple choice self-assessment. The course is designed for online classes, hybrid classes, blended classes and asynchronous delivery of content. All of this information is included in an Energy Diagram. Potential energy. Reaction Coordinate. Starting material. Transition states. Transition.39 pages
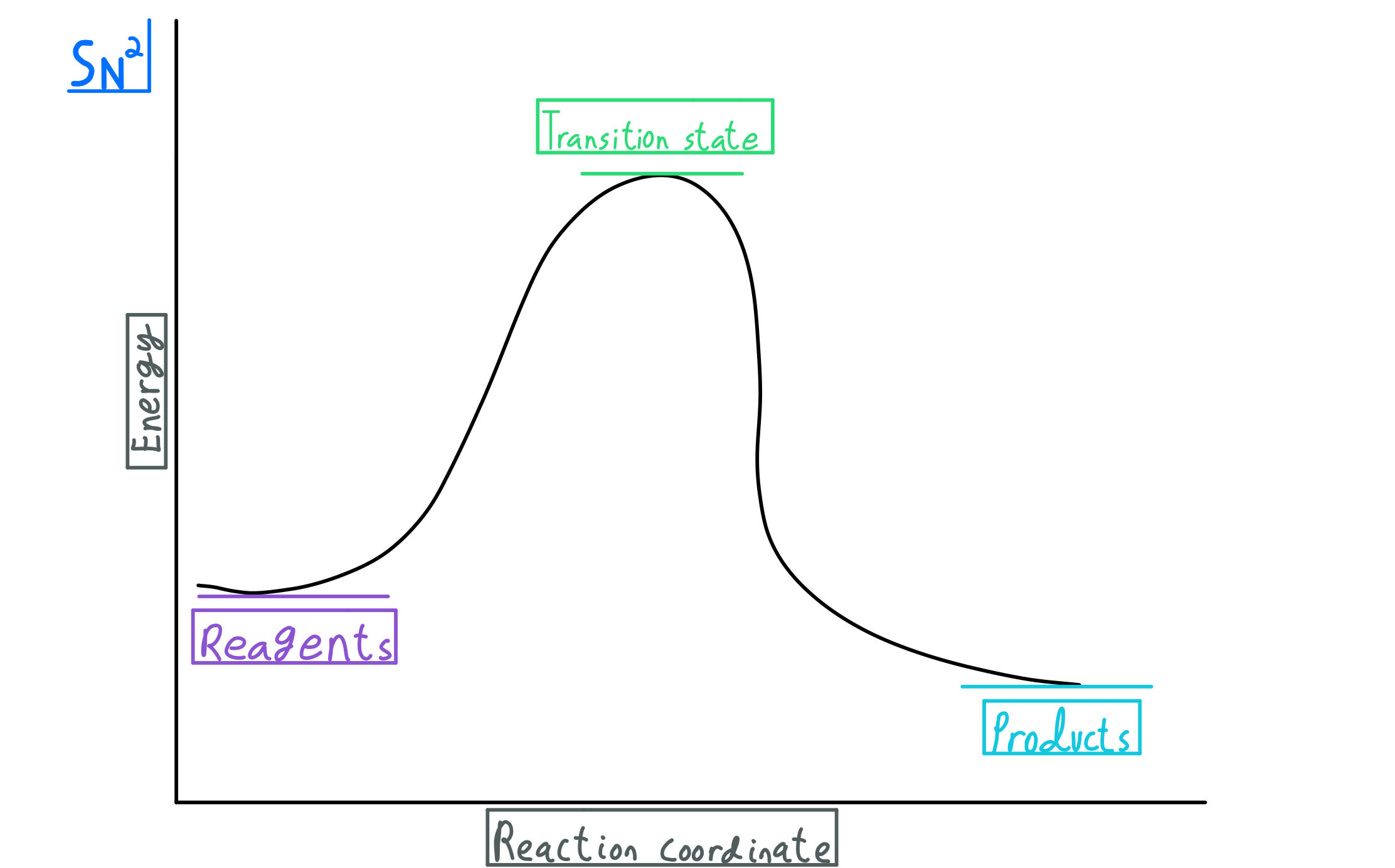
A rearrangement reaction is a board class of organic reaction where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule.Often a substituent moves from one atom to another atom in the same molecule. One rearrangement pathway where an unstable carbocation can be transformed into more stable carbocation is called hydride shift. Lecture handouts. LEC # TOPICS LECTURERS HANDOUTS; 1. Introduction to Organic Chemistry, Review, Molecular Structure. Dr. Kimberly Berkowski ((2. Resonance, Acidity and Basicity Kinetics | Organic Chemistry 1: An open textbook. 5.4. Kinetics. Now, let's move to kinetics. Look again at the energy diagram for exergonic reaction: although it is 'downhill' overall, it isn't a straight downhill run. First, an 'energy barrier' must be overcome to get to the product side. The height of this energy barrier, you may ... reaction coordinate. The reaction coordinate indicates how far the reaction has progressed, from no reaction to a completed reaction. Figure 5.1 shows an energy diagram for the reaction of C AJB to form C JA B. This reaction occurs in one step, meaning that bond breaking in reactants and bond form-ing in products occur simultaneously.
You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the 'reaction coordinate', tracing from left to right the progress of the reaction from starting compounds to final products.
In this video, Dr. Norris goes over practice problems in interpreting reaction energy coordinate diagrams.
For full-year courses in organic chemistry taken by science and pre-health professions majors. ... 3.8 Using a Reaction Coordinate Diagram to Describe a Reaction . 4. THE REACTIONS OF ALKENES . 4.1 Addition of a Hydrogen Halide to an Alkene.
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
As chemists we want to know the mechanism of this reaction! (or how does the energy diagram appear)! What we know:! Reaction does not proceed in the dark or in the cold! Reaction occurs with wavelengths corresponding to Cl 2 absorption! Quantum yield is greater than 1! (therefore more moles of product are obtained than moles of photons of light ...
Jun 9, 2018 — Organic chemistry students struggle with understanding the energetics of chemical reactions. Reaction coordinate diagrams are one tool that ...
The reaction coordinate diagram shows that the energy of activation for the reverse reaction is lowered by the catalyst as well. Enzymatic catalysis The ability of enzymes to catalyze reactions depends on their ability to interact directly and specifically with reactants The reactant of an enzyme-catalyzed reaction is termed the substrate .
For a chemical reaction or process an energy profile (or reaction coordinate diagram) is a theoretical representation of a single energetic pathway, along the reaction coordinate, as the reactants are transformed into products. Reaction coordinate diagrams are derived from the corresponding potential energy surface (PES), which are used in computational chemistry to model chemical reactions by ...
Reaction Coordinate Diagram of Ozone Photolysis The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction . Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.
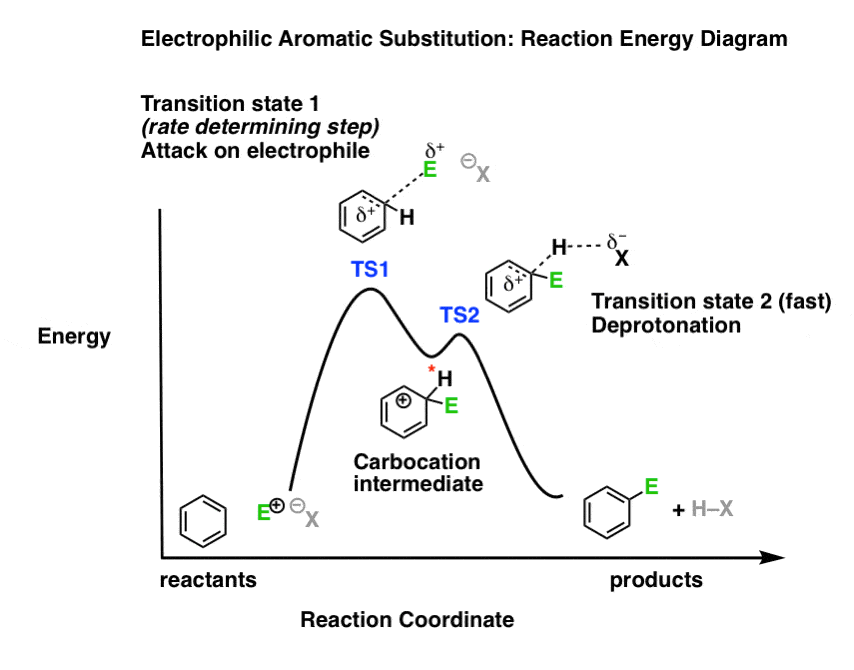
Diagram shows an energy diagram tracing the progress of a reaction that occurs by an S N 1 mechanism. The rate of the reaction reflects the activation energy required to form the carbocation intermediate. The activation energy required for step 2, addition of the nucleophile to the carbocation, is much smaller, so step 2 is very fast.
X Exclude words from your search Put - in front of a word you want to leave out. For example, jaguar speed -car Search for an exact match Put a word or phrase inside quotes.
Reaction Coordinate Diagram Which Reaction Coordinate Diagram Represents A Reaction In Which The Activation Energy Ea Is 50 Kjmol 1 And The Hrxn Is 15 Kj Mol 1. Reaction Coordinate Diagram Illustrated Glossary Of Organic Chemistry Reaction Coordinate. Reaction Coordinate Diagram Illustrated Glossary Of Organic Chemistry Rate Determing Step.
5.3: Reaction coordinate diagrams. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of ...
Organic chemistry students struggle with understanding the energetics of chemical reactions. Reaction coordinate diagrams are one tool that is widely used in organic chemistry classrooms to assist students with visualizing and explaining the energy changes that take place throughout a reaction.
Illustrated Glossary of Organic Chemistry Rate determining step (rds; rate limiting step): The mechanism step with the greatest activation energy (i.e., the slowest step) and therefore the step that has the greatest influence on reaction rate .
energy diagram (or reaction energy diagram): a graph of the energy of a reaction against the progress of the reaction. enthalpy: a thermodynamic state function, generally measured in kilojoules per mole. In chemical reactions the enthalpy change (deltaH) is related to changes in the free energy (deltaG) and entropy (deltaS) by the equation:










.gif?revision=1&size=bestfit&width=960&height=720)







0 Response to "40 reaction coordinate diagram organic chemistry"
Post a Comment