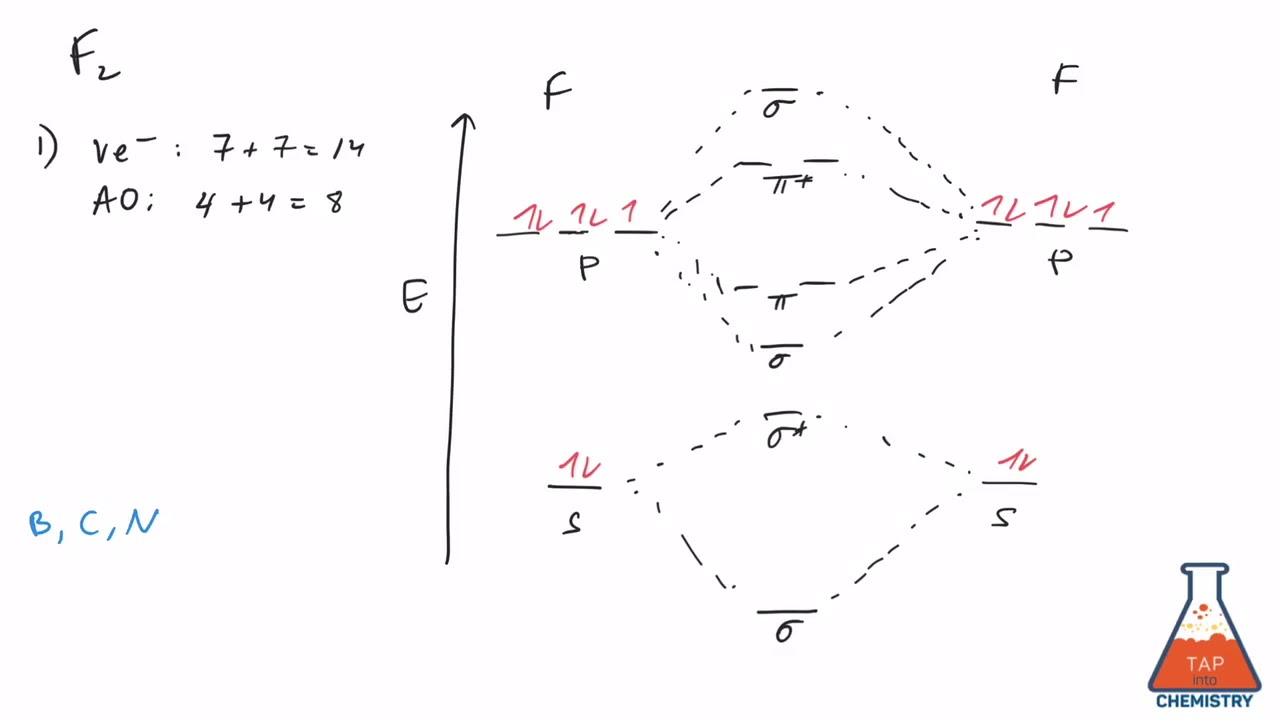
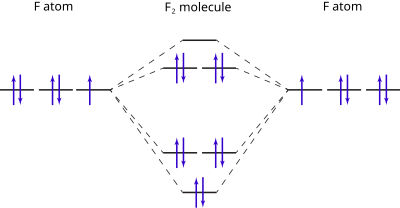
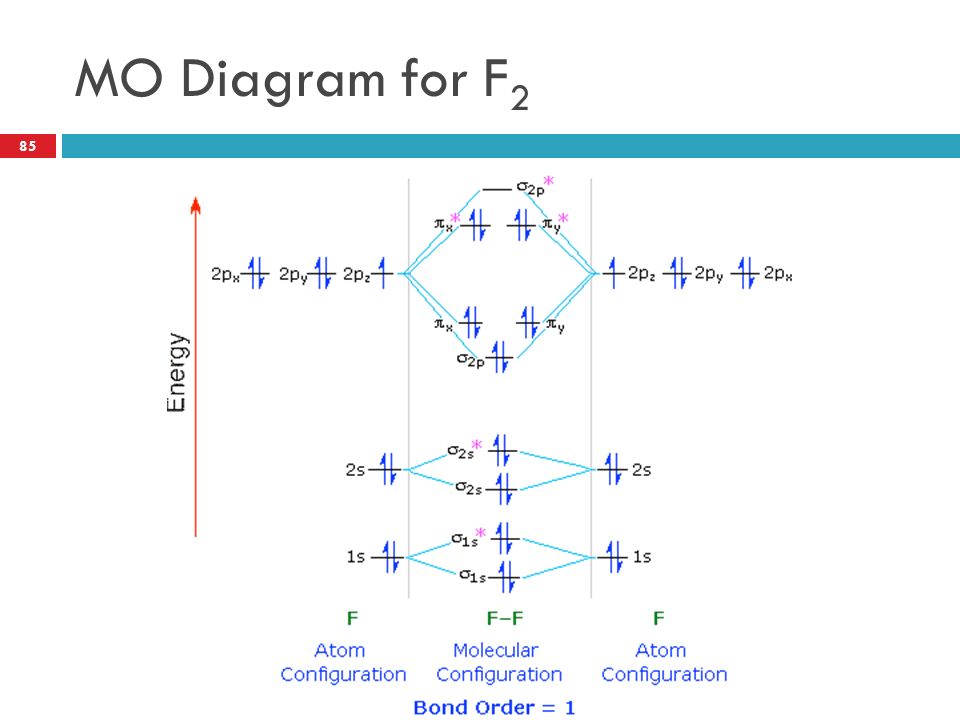
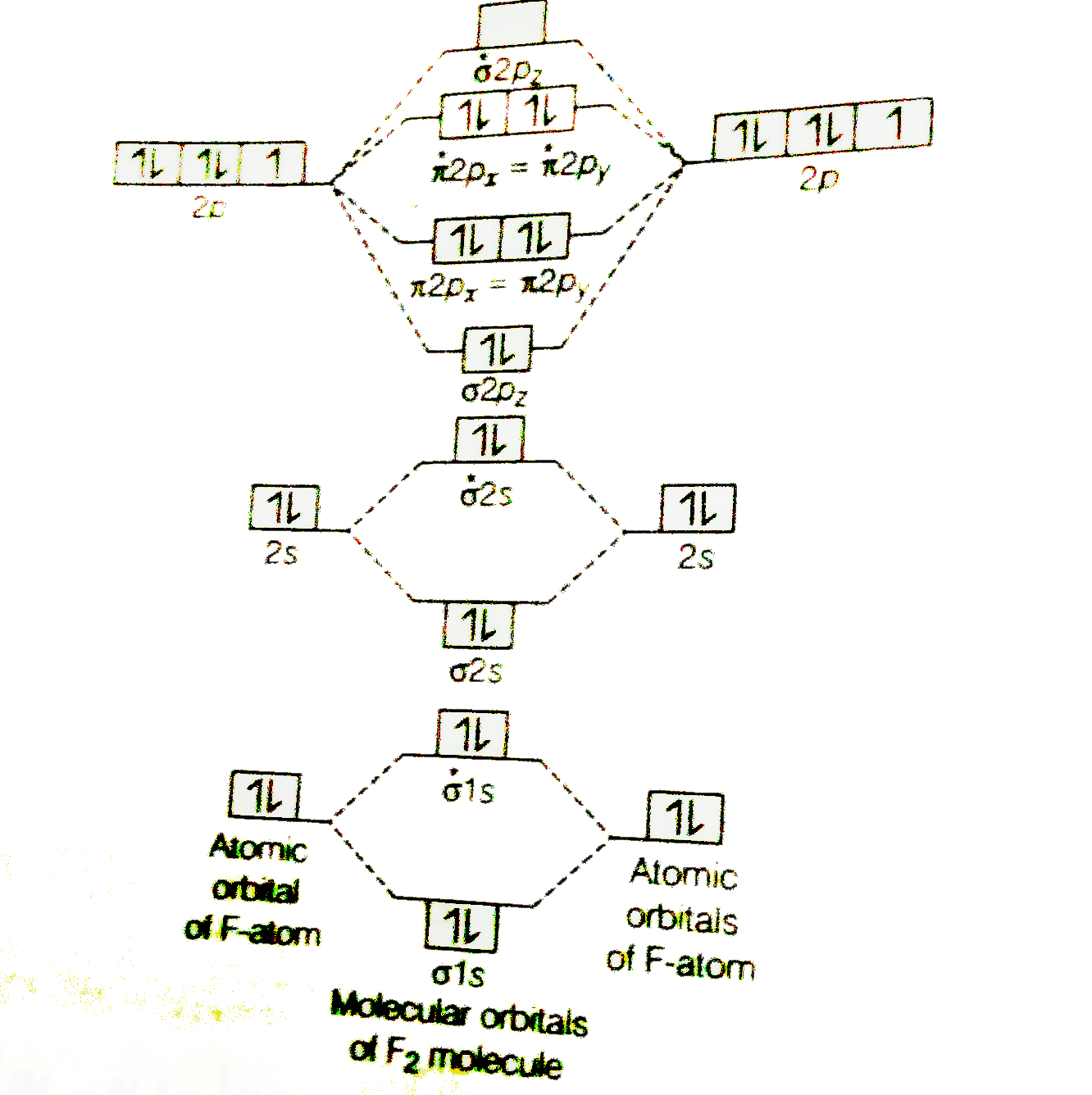
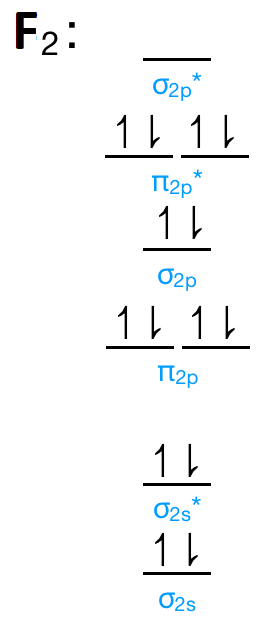
42 mo diagram of f2
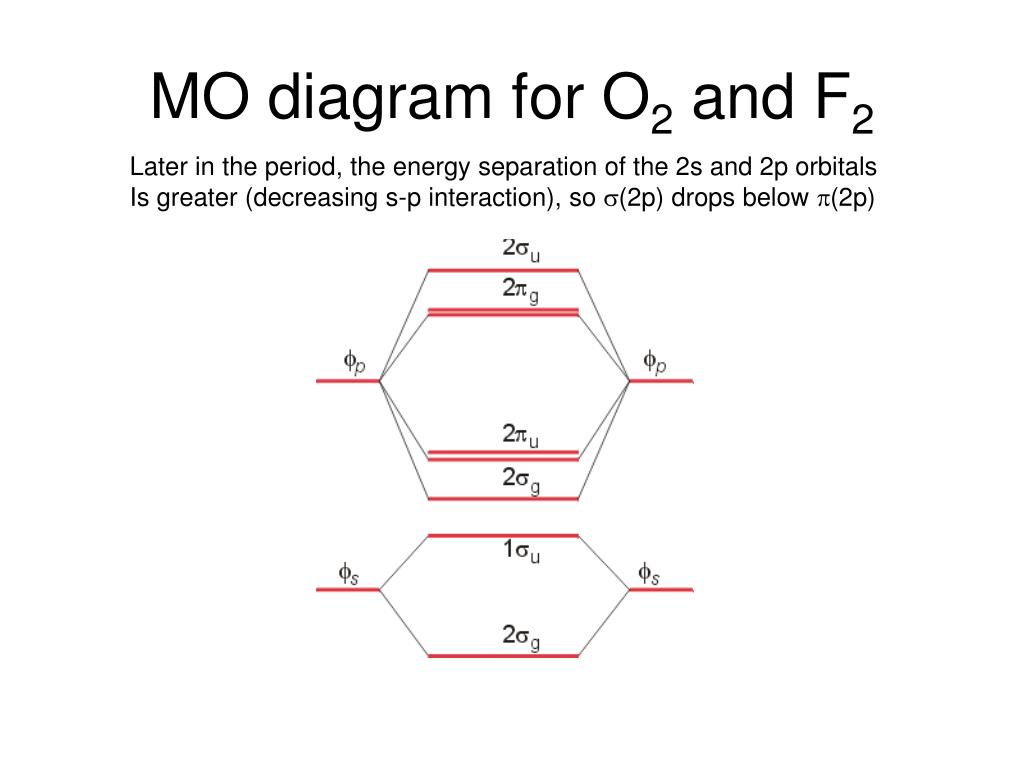
this diagram explains the observed paramagnetism of B2. Figure 9.39: The molecular orbital energy-level diagrams, bond orders, bond energies, and bond lengths for the diatomic molecules B 2 through F2.Note that for O2 and F2 the σ2p orbital is lower in energy than the π2p orbitals. 3.O2 has 12 valence electrons. Its MO configuration is: 1. Draw an MO diagram for the valence electrons in F2. What is the bond order? How many o and a bonds are there? What is the HOMO and LUMO? What is the magnetism of the species? Question: 1. Draw an MO diagram for the valence electrons in F2. What is the bond order? How many o and a bonds are there? What is the HOMO and LUMO?
mo bonding in f2 and o2 chemistry libretexts molecular orbitals mo are constructed from atomic orbitals in o 2 and f 2 there is a crossover of the sigma and the pi ortbials the relative energies of the sigma orbitals drop below that of the pi orbitals information from the mo diagram justify o2 s stability and show that it s bonding order is 2.
Mo diagram of f2
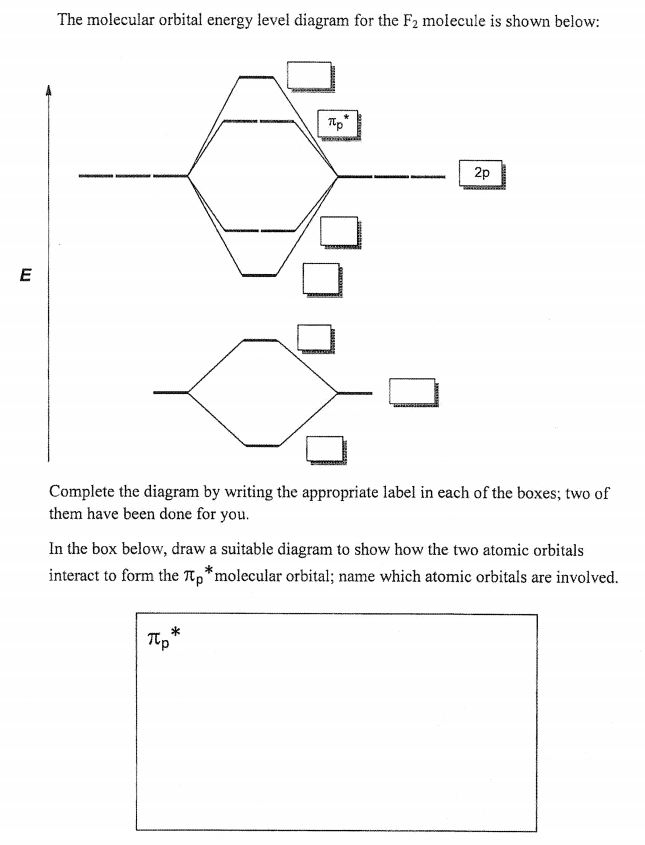
F2 Molecular Orbital (MO) Diagram. As per molecular orbital (MO) theory, all the constituent atoms in a molecule contribute to the formation of molecular orbitals. These MOs are a linear combination of the atomic orbitals. Thus, the electrons in a molecule are not individually assigned to atomic orbitals but to molecular orbitals. Let us have a ... A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H
Mo diagram of f2. Draw molecular orbital diagram for F2 molecule. Also, give its electronic configuration, bond order and magnetic property. 138. Solve the following: Open in App. Solution. Verified by Toppr. Solve any question of Chemical Bonding and Molecular Structure with:-Patterns of problems. Molecular orbital diagram for c2. This video shows the mo diagrams of the c2 n2 o2 and f2 molecules. Molecular orbitals are formed combining similar atomic orbitals. Just because some chemical species shows integral value of bond order doesnt mean that it should exist. Molecular orbital diagram for the molecule oxygen o2. Answer (1 of 6): Here is the solution, > * For O2 molecule, > * For F2 molecule, Thanks for reading. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
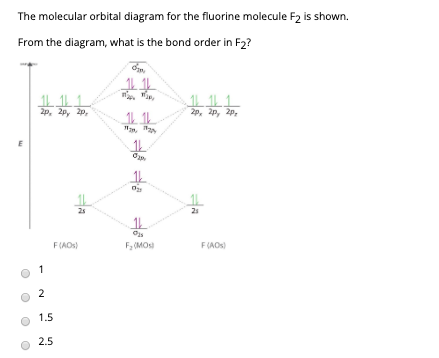
Molecular orbital diagram for f2. A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same number of. C would this ion exist. To further demonstrate the consistency of the lewis structures with mo. For the ion f2. Draw molecular orbital diagram for F 2 molecule. Also, gives its electronic configuration, bond order and magnetic property. Hint: The Molecular Orbital Theory (MOT) explains the formation of the molecule in a better way than Valence Bond Theory (VBT). The bond order calculations are feasible using MOT and so is the description of electronic ... Setting up the diagram Start by considering the axial definition: Always put the z-axis along the bond in diatomics. Always add a diagram clearly showing how the axial system related to your molecule on your MO diagram then start the diagram itself, remember the vertical "axis" of the whole diagram is energyand the horizontal axis are For example, an ns/ns overlap for a homonuclear diatomic molecule gives rise to a partial MO diagram like this: and an np/np overlap for O2 and F2 gives: So, the full MO diagram is: Thus, the valence electron configuration is: (σ2s)2(σ* 2s)2(σ2pz)2(π2px)2(π2py)2(π* 2px)2(π* 2py)2. Answer link.
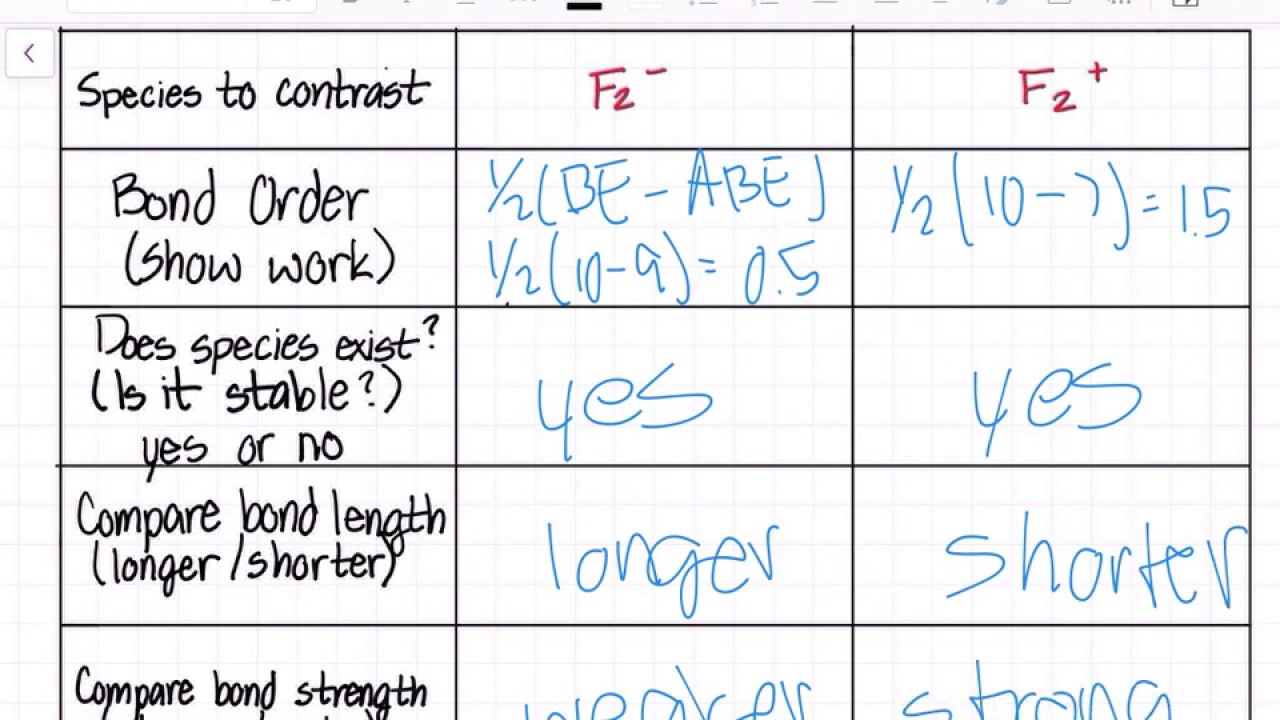
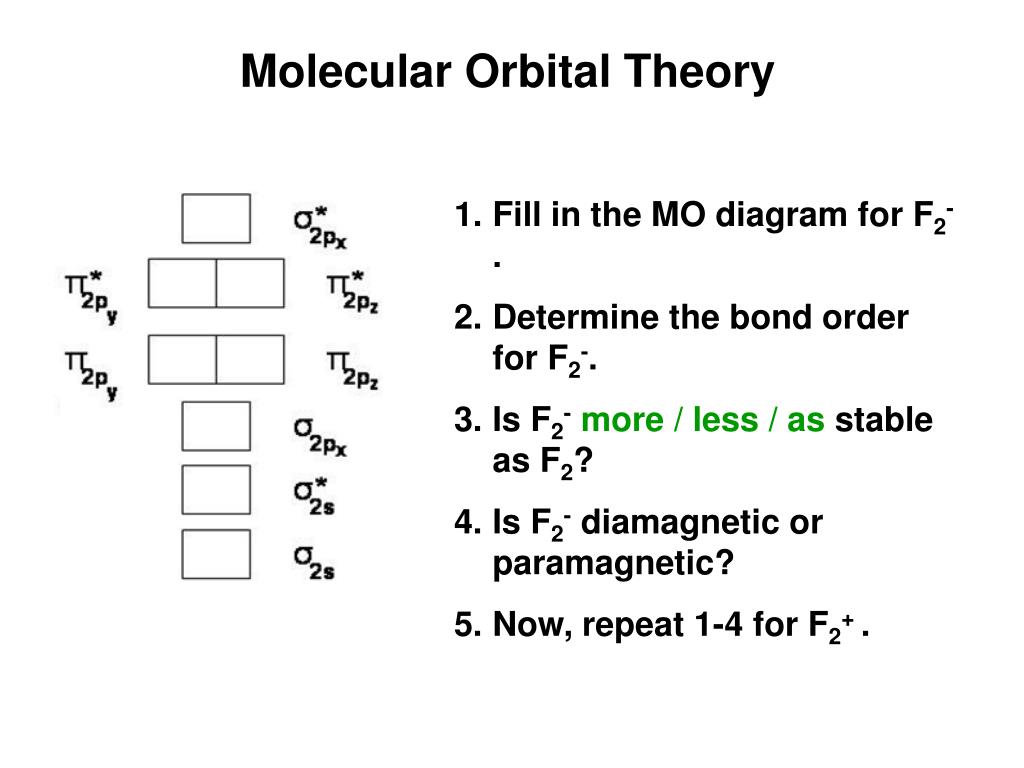
Valence electrons in F2- = 15 It's molecular orbital confirguation will be Bond order = (Bonding electron - antibonding electron)/2 = 8-7/2 = 0.5 bond order No of sig …. View the full answer. Transcribed image text: 2. Draw an MO diagram for the valence electrons in the F2 ion. What is the bond order? Molecular orbital diagram of N 2 BO = [Nb-Na] = [10-4] = 3 Since all the electrons in nitrogen are paired, it is diamagnetic molecule. Answered by | 13th Jun, 2016, 04:45: PM. Concept Videos. Molecular Orbital Theory - Part 1. MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine Molecular Orbital Diagram - Cl2, Br2, I2 3s & 3p and higher atomic orbitals are not so widely separated in energy and allow significant mixing (hybridization) to occur. This mixing causes the inversion of the σσand πmolecular orbitals' energy. σσσ ππ σ* π* 3,4,5 p 3, 4,5 s σ* σ 3,4,5 s 3,4,5 p Interhalogens Br Br F F Br F F F F.
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. Molecular orbital diagram for f2. The other molecular orbital produced s h h shows a decrease in electron density between the nuclei reaching a value of zero at the midpoint between the nuclei where there is a nodal plane.
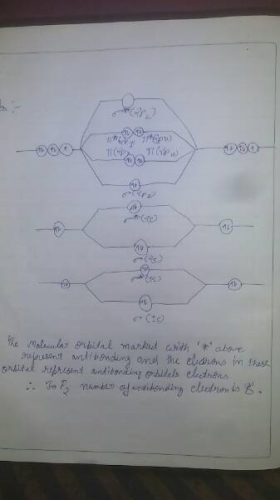
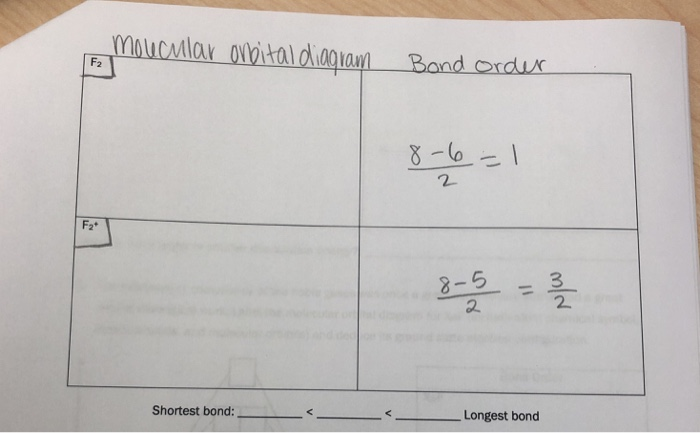
Arrange the following in order of decreasing stability. a blank molecular orbital diagram (part a 1 figure) has been provided to you. rank the fluorine species from most to least stable. to rank items as equivalent, overlap them. f2, f2+, f2-
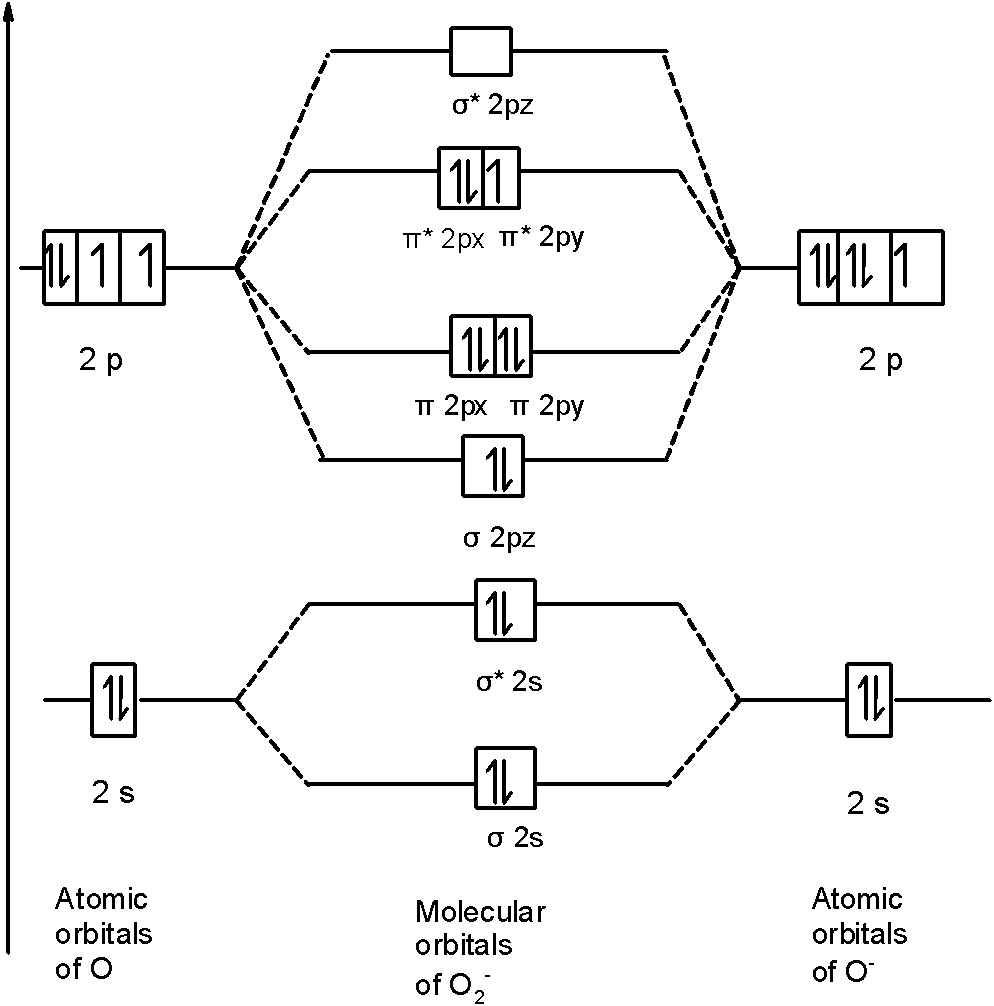
The valence electrons = 14; BO = 0.5* (8-6) = 1. The bond order is commonly used to signify the bond stability. Higher bond order indicates more stability and vice versa. Thus, is the most stable. is diamagnetic while and are paramagnetic in nature. Further Explanation: MO diagram: It is a tool used to describe the chemical bonding formed ...
#3. Draw the MO diagram for `O_2^+` This is a bit of a curveball, but a perfectly valid problem. Recall that a cation indicates a loss of `1` electron. `O_2^+` is just the ionized form of `O_2`; that is, it's `O_2` with `1` missing electron. The MO diagram will be the same as the MO diagram of `O_2`, except with `1` less electron.
Now let's put these ideas together to make an MO diagram for HF. We need to know what orbitals we are using. We are only going to consider valence orbitals. H has a 1s orbital. F has a 2s orbital and 3 2p orbitals (x,y,z). We want to know the energies of the orbitals.
Exercise 3.3.4. 3. Construct a qualitative molecular orbital diagram for chlorine, Cl 2. Compare the bond order to that seen in the Lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). Answer.
Answer (1 of 4): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th...
Identify the MO diagram for F2. F2 valence e−: diagram A 6 diagram A 8 diagram A 10 diagram B 12 diagram B 14 Solution In B2, C2, and N2, the 𝜎2𝑝 orbital is higher in energy than the 𝜋2𝑝 orbitals as shown in diagram A.
This video is about MO Diagram #2 - F2
When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so ...
As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond. This example was covered in class to show the rare exception that this single bond is a bond.
Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
F2 Molecular Orbital (MO) Diagram. As per molecular orbital (MO) theory, all the constituent atoms in a molecule contribute to the formation of molecular orbitals. These MOs are a linear combination of the atomic orbitals. Thus, the electrons in a molecule are not individually assigned to atomic orbitals but to molecular orbitals. Let us have a ...

Write The Molecular Electronic Configuration Of F2 And C2 Draw The Energy Level Diagram Calculate Brainly In



















0 Response to "42 mo diagram of f2"
Post a Comment