42 orbital diagram of titanium
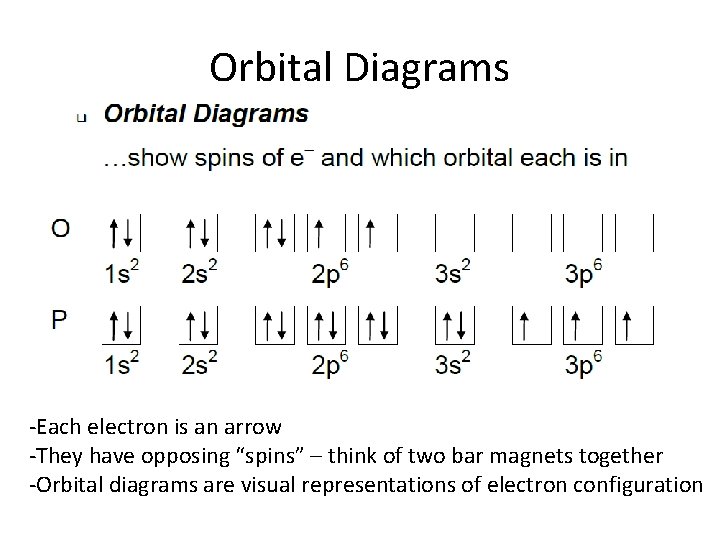
Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ↿ ↿ 4s ↿⇂ 4p 4d 4f: ... Titanium atoms have 22 electrons and the shell structure is 2.8. 10.2. The ground state electron configuration of ground state gaseous neutral titanium is [Ar]. What element has the electron configuration of 1s2 2s2 ... Exam 4 Review: Ch.8-9. Electromagnetic radiation with a wavelength of 745 nm appears as red light to the human eye. The energy of one photon of this light is ________ J. Calculate the wavelength (in nm) of the blue light emitted by a mercury lamp with a frequency of 6.19 × 10^14 Hz. Nice work!
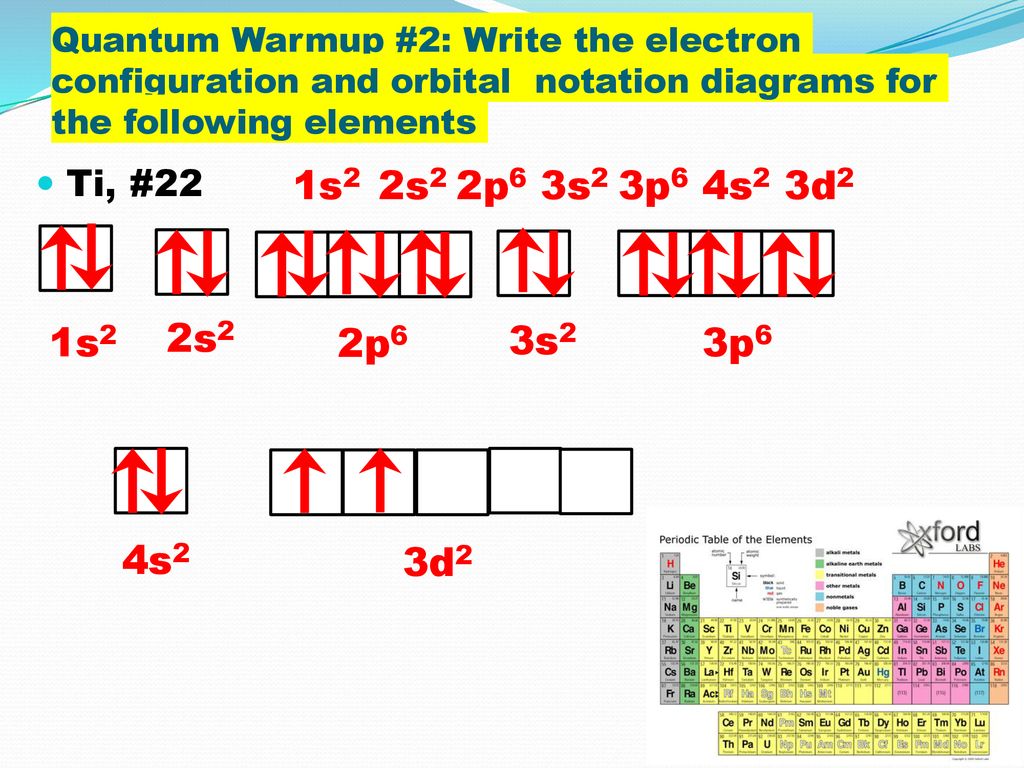
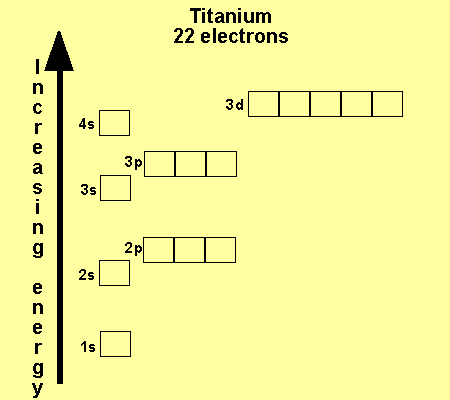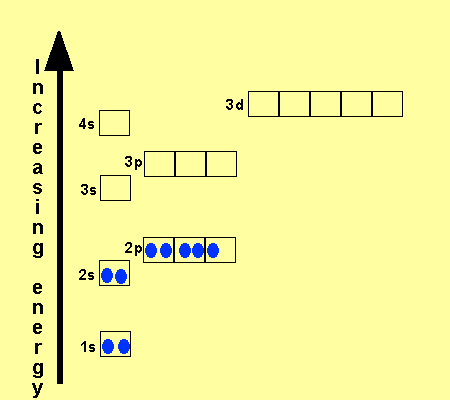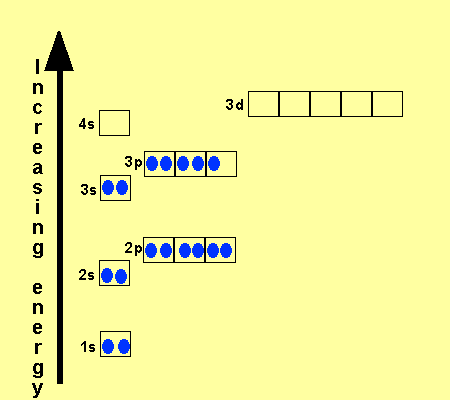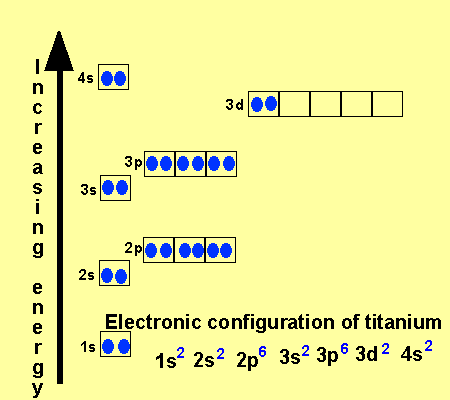
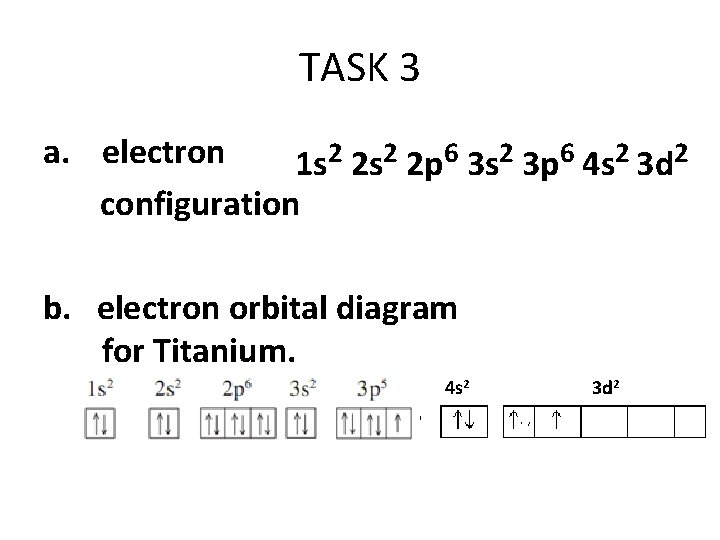
41 orbital diagram for titanium. The electron configuration for titanium is 1s22s22p63s23p63d24s2, according to the Jefferson Lab website. The element's 22 electrons are arranged in four energy levels surrounding the nucleus of the atom. Electrons orbit the nucleus in energy levels, which are also called shells.

Orbital diagram of titanium
Orbital Diagram For Titanium. First you'd follow the filling of orbitals in accordance with the Aufbau principle. This video shows how to draw the orbital diagram of Titanium (Ti). Orbital Diagram For Ti2 — UNTPIKAPPS (Nicholas Hudson) Fill in the electron configurations for the elements given in the table. orbital #4s, so you can see here However, once the #4s # orbital is filled, it becomes higher in energy than the orbital#3d #. This means that when titanium loses electrons, it does so from #4s # orbital first. #Ti: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^2 4s^2# Therefore, the two electrons that get lost when Titanium exists as several isotopes. The mass spectrum of a sample of titanium gave the data : Calculate the relative atomic mass of titanium to two decimal places. Ans ... Sketch the orbital diagram of the valence shell of a bromine atom (ground state) on the energy axis provided.
Orbital diagram of titanium. Element reactions. Titanium atoms have 22 electrons and the shell structure is 2.8.10.2. The ground state electron configuration of ground state gaseous neutral titanium is [ Ar ]. 3d2. 4s2 and the term symbol is 3F2. Schematic electronic configuration of titanium. The Kossel shell structure of titanium. Oxygen electron configuration is 1s 2 2s 2 2p 4.The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen(O) and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles. The eighth element in the periodic table is oxygen. All right, so we just did scandium and titanium. All right, so scandium was argon 4s 2, 3d 1. We talked about two electrons in the 4s orbital, one electron in the 3d orbital. We just did titanium 4s 2, 3d 2 or once again you could switch any of these. When you're doing orbital notation, adding that second electron to a d orbital. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
Hey, guys, Welcome back. All right, So here, we to construct orbital diagrams for each of these, um, Adam or irons. So we have titanium ...Jan 21, 2019 That happens because the empty #3d# orbitals are actually higher in energy than the empty #4s# orbital, as seen here. However, once the #4s# orbital is filled, it becomes higher in energy than the #3d# orbitals. This means that when titanium loses electrons, it does so from the #4s# orbital first. #"Ti: " 1s^2 2s^2 2p^6 3s^2 3p^6 3d^2 4s^2# The electron configuration for titanium is 1s22s22p63s23p63d24s2, according to the Jefferson Lab website. The element's 22 electrons are arranged in four energy levels surrounding the nucleus of the atom. Electrons orbit the nucleus in energy levels, which are also called shells. These energy levels contain sub-shells, or orbitals, each of ... This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble...
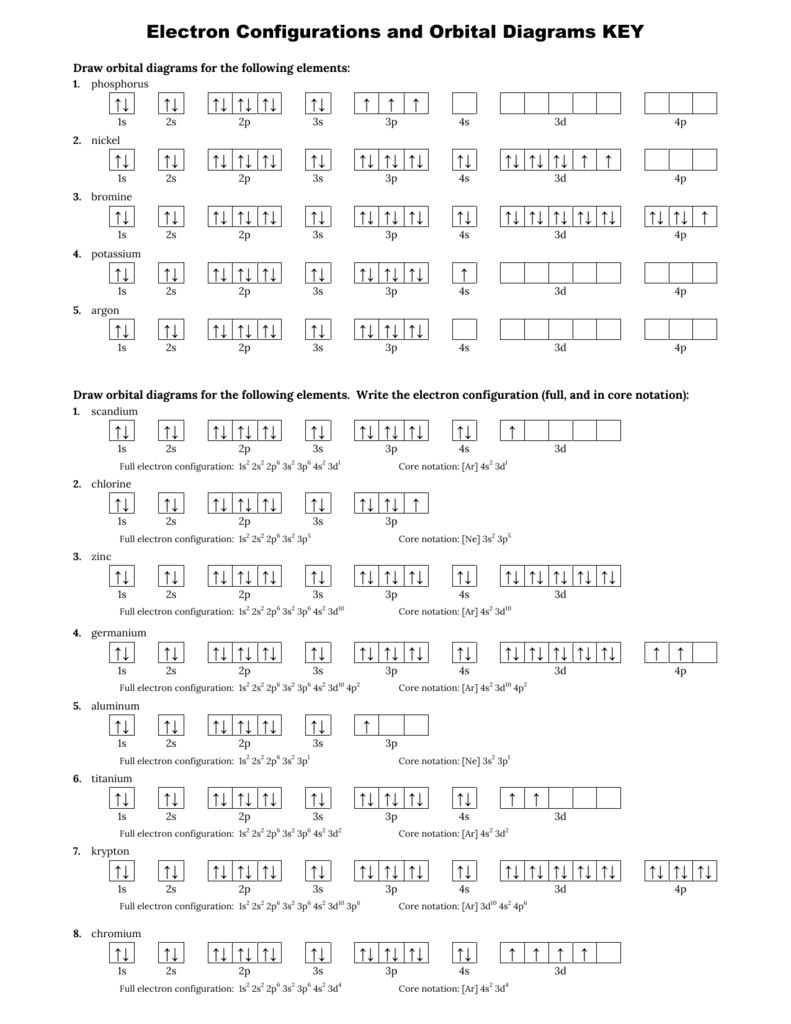
Orbital notation shows the number of electronics in an orbit. The orbital notation of Hydrogen is a circle with one slash through it. The electron configuration of Hydrogen is 1(s^1). Titanium has 1s, 2s, 2p, 3s ,3p, 3d, 4s orbitals in which electrons are present. The valence electrons present in valence shells, 3d and 4s are 4. These ...1 answer · Top answer: Titanium is a transition element which has atomic number 22. It forms various types of compounds like titanium tetrachloride and trichoride etc. It is popularly ... Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium ... Titanium Electron Configuration (Ti) with Orbital Diagram. Titanium Electron Configuration: Titanium is a chemical element that has a chemical symbol Ti. Its atomic number is 22. It is a transition metal lustrous which has a silver colour, low density, and high strength. It is resistant to corrosion in aqua regia, sea water, and chlorine.

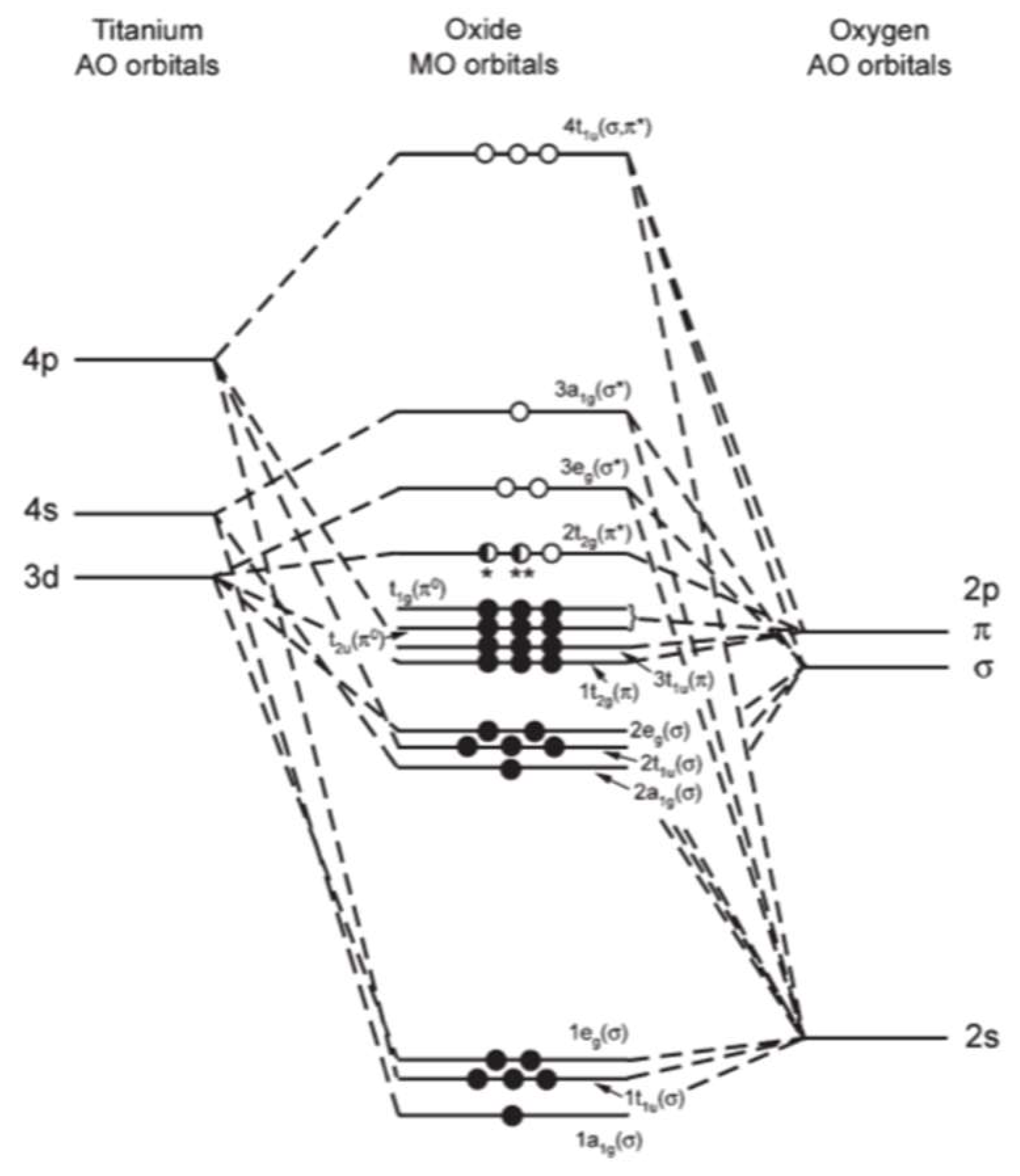
Figure 5 From Tri Tert Butylsily 1 Imido Complexes Of Titanium Benzene C H Activation And Structure Of Semantic Scholar
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
The B 3&Pgr;–X 3Δ (1,0) band of titanium monoxide has been studied at sub-Doppler resolution (0.002 cm−1) by crossing a beam of TiO molecules with a cw ...
Catalysts Free Full Text Switchable Intrinsic Defect Chemistry Of Titania For Catalytic Applications Html
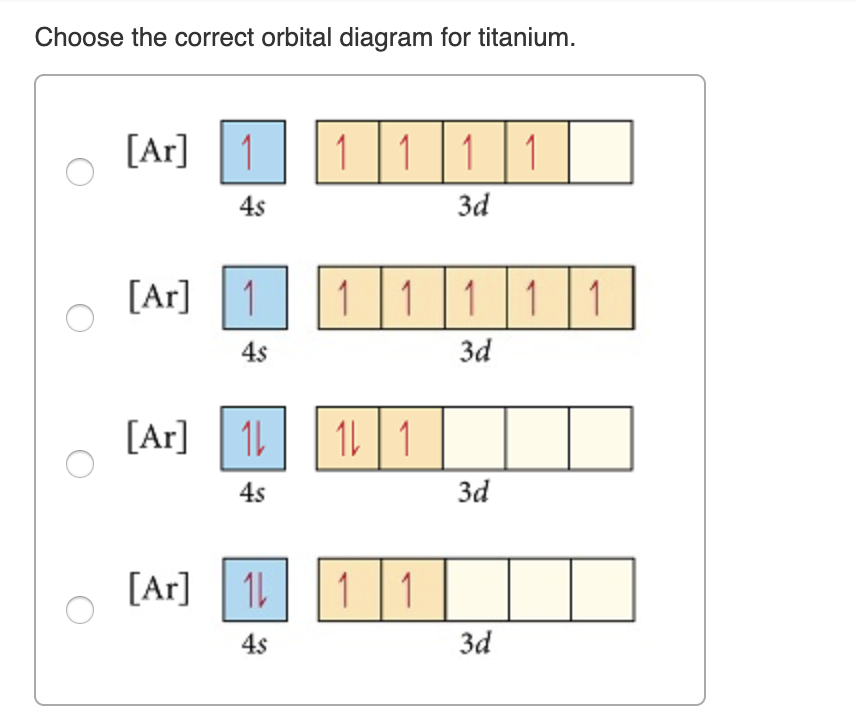
on Orbital Diagram For Ti2+. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? The probability of finding an electron at the nucleus is 0. However, once the 4s orbital is filled, it becomes higher in energy than the 3d orbitals. This means that when titanium loses electrons, it does so.
Lecture 11 { Spin, orbital, and total angular momentum MATH-GA 2710.001 Mechanics 1 Very brief background In 1922, a famous experiment conducted by Otto Stern and Walther Gerlach, involving particles subject to a nonuniform magnetic eld, identi ed two surprising properties of the angular momentum: 1) the components
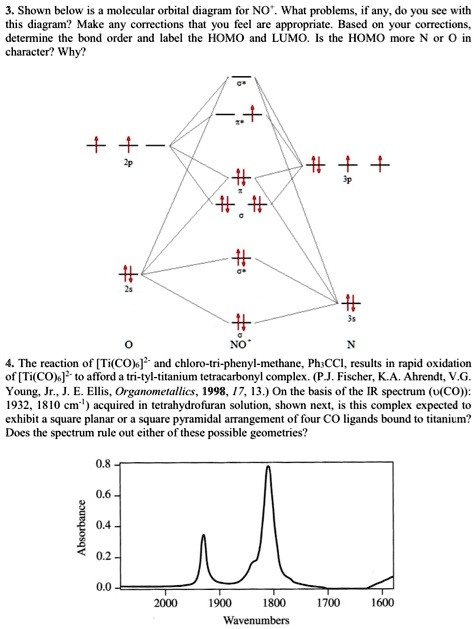
Solved Shown Below Is Molecular Orbital Dixgram For No What Problems If Any Do You See With This Diagram Make Any Coneclions Thal You Ecl We Appropriate Based Youi Comecane Determine The
Let's consider titanium (Z = 22). Its electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 2, which the (n + l) rule correctly predicts. If the electron configuration depended solely on the orbital energies, we would expect: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 4 - with no electrons in the 4s orbital.
Orbital Diagram. 1s ... Titanium dioxide (TiO2), a white pigment that covers surfaces very well, is used in paint, rubber, paper and many others. Sources Usually occurs in the minerals ilmenite (FeTiO3) or rutile (TiO2). Also in Titaniferous magnetite, titanite (CaTiSiO5), and iron ores. Pure metal produced by heating TiO2 with C and Cl2 to ...
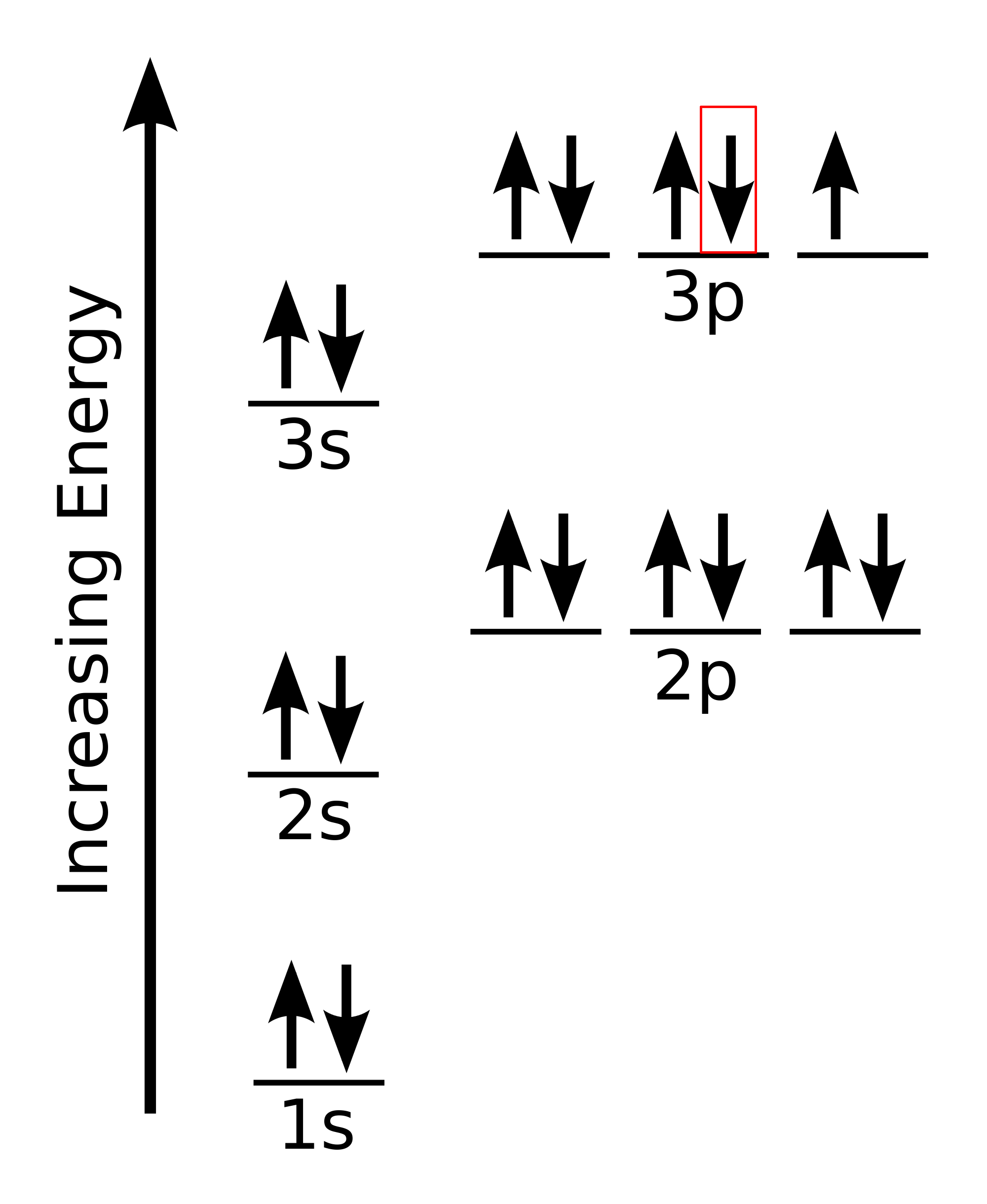
What Is A Set Of Four Quantum Numbers That Could Represent The Last Electron Added Using The Aufbau Principle To The Cl Atom Socratic
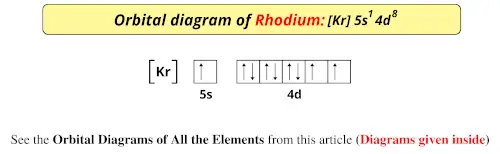
Orbital Diagram For Chromium Electron Configurations In The 3d Orbitals. Orbital Diagram For Chromium Exam 2013 Chem1010 Fundamentals Of Chemistry Studocu. Orbital Diagram For Chromium Give Electronic Configuration And Orbital Diagram Of Titanium And
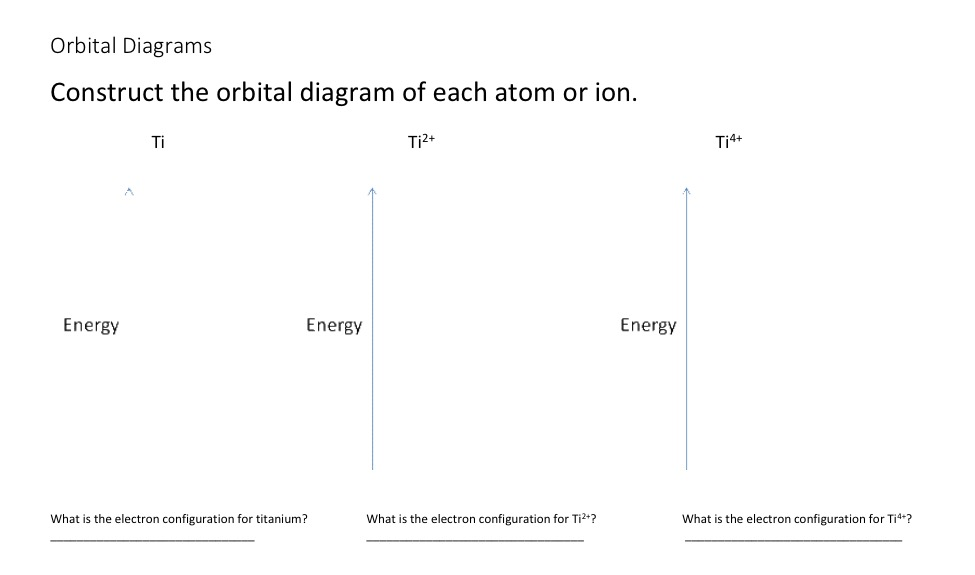
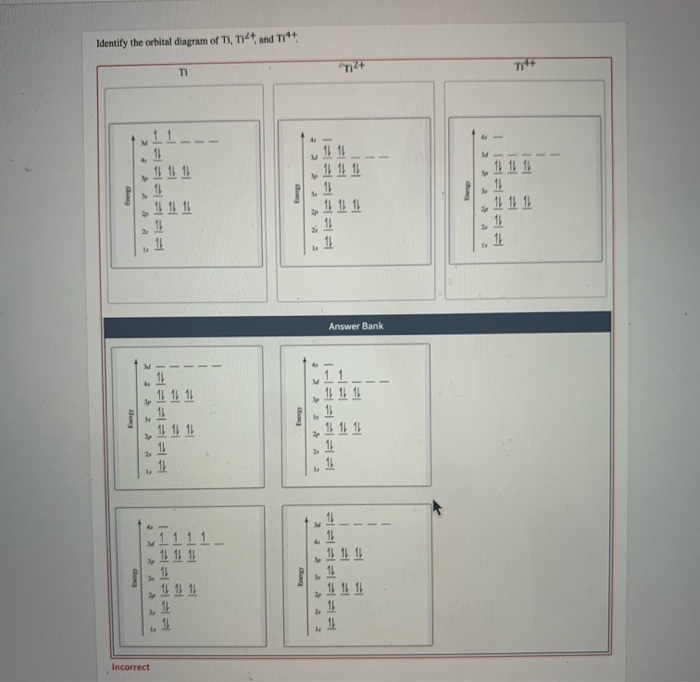
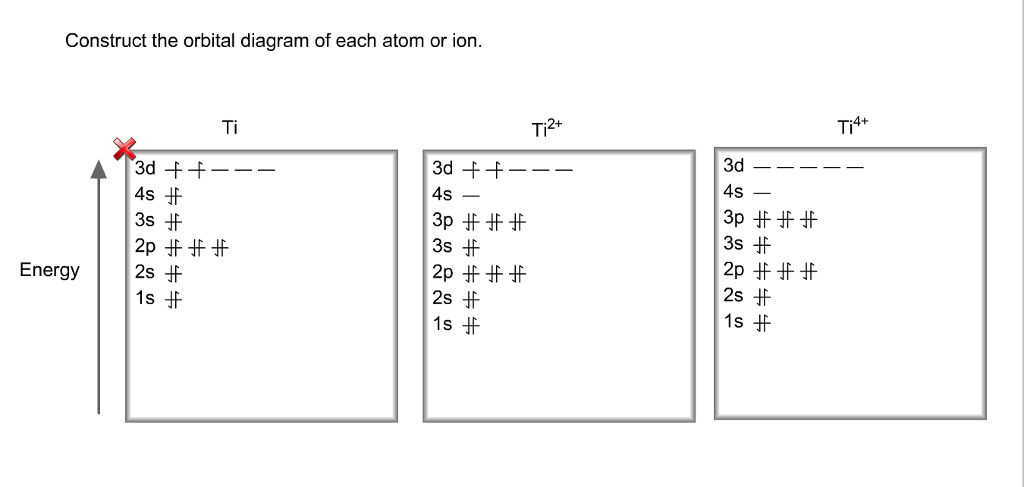
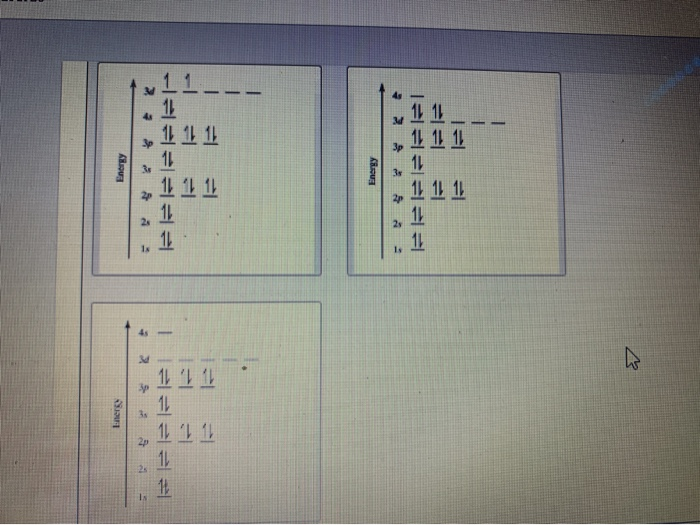
Get the detailed answer: What is the orbital diagram of each atom or ion? Ti, Ti2+, Ti4+
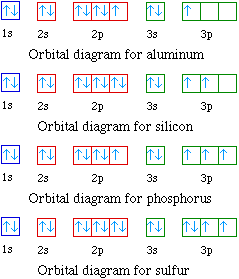
Choose the orbital diagram that represents the ground state of N. orbital diagram where 1s and 2s orbitals contain 1 pair of electrons each. 2p orbitals are empty. orbital diagram where 1 s and 2 s orbitals contain 1 pair of electrons each. 2 p orbitals contain 3 pairs of electrons.
Titanium atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Check out the blackboard. That box on the left has all of the information you need to know about one element. It tells you the mass of one atom, how many ...

Draw An Orbital Diagram Showing Valence Electrons And Write The Condensed Ground State Electron Brainly Com
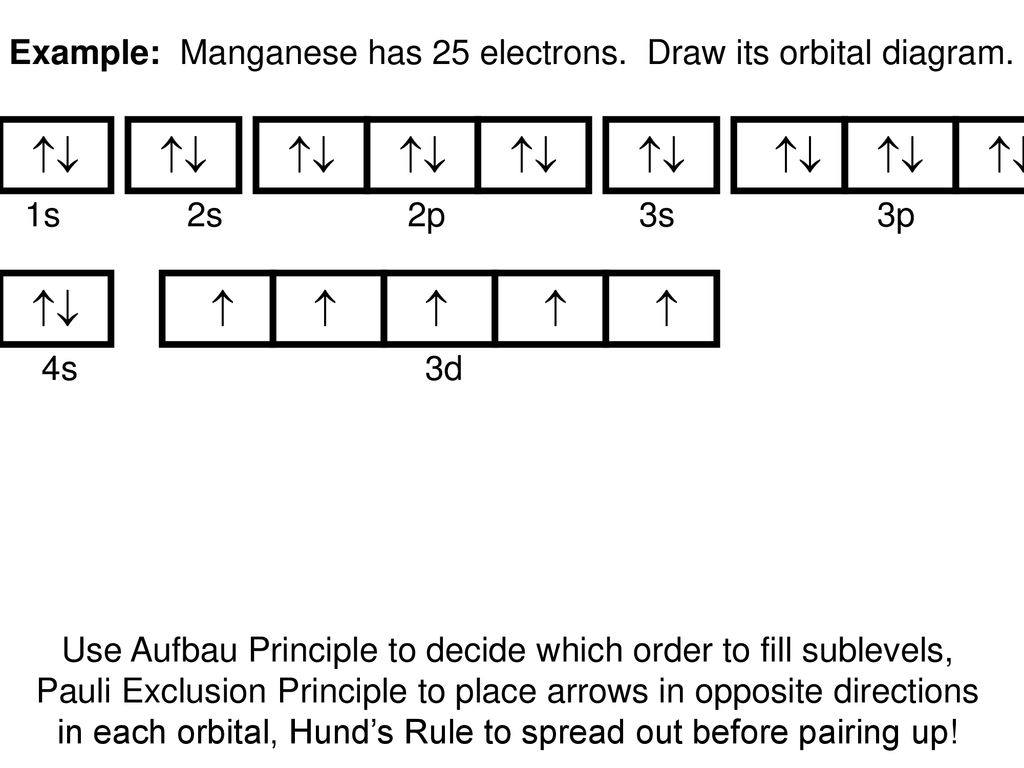
Hund's rule: Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of the single electrons must have the same spin. orbital filling diagram: A visual way to represent the arrangement of all the electrons in a particular atom.
For that, we have electron shell diagrams. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. For each electron shell atom diagram, the element symbol is listed in the nucleus. This is a Bohr Diagram of a titanium atom. The nucleus of a titanium atom has 22 protons and 26 neutrons.
Answer (1 of 2): First you'd follow the filling of orbitals in accordance with the Aufbau principle. For titanium you have 22 electrons to account for as follows: * 1s2 = 2 electrons giving a total of 2 * 2s2 = 2 electrons giving a total of 4 * 2p6 = 6 electrons giving a total of 10 * 3s2 =...
Titanium exists as several isotopes. The mass spectrum of a sample of titanium gave the data : Calculate the relative atomic mass of titanium to two decimal places. Ans ... Sketch the orbital diagram of the valence shell of a bromine atom (ground state) on the energy axis provided.
orbital #4s, so you can see here However, once the #4s # orbital is filled, it becomes higher in energy than the orbital#3d #. This means that when titanium loses electrons, it does so from #4s # orbital first. #Ti: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^2 4s^2# Therefore, the two electrons that get lost when
Orbital Diagram For Titanium. First you'd follow the filling of orbitals in accordance with the Aufbau principle. This video shows how to draw the orbital diagram of Titanium (Ti). Orbital Diagram For Ti2 — UNTPIKAPPS (Nicholas Hudson) Fill in the electron configurations for the elements given in the table.

Electron Orbital Diagram Of Titanium Diagram Base Website Of Atom Diagrams Showing Electron Shell Configurations Of The Elements


















0 Response to "42 orbital diagram of titanium"
Post a Comment