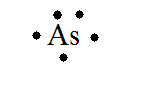
44 arsenic electron dot diagram
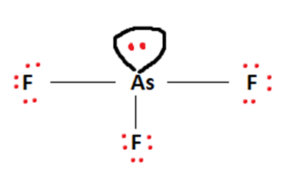
AsF5 lewis structure is made up of one Arsenic atom situated in a central position and five fluorine atoms that spaced evenly around the central atom. There is a total of 10 bonding electrons and 30 nonbonding electrons present in the lewis structure of AsF5. arsenic (As) strontium (Sr) krypton (Kr) gallium (Ga) tin (Sn) sulfur (S) strontium (Sr) and gallium (Ga): have fewer than four dots in their electron dot diagrams. s
Arsenic pentoxide is used as a solid or as a solution in the manufacturing of arsenates, weed killer, metal adhesives, insecticides, fungicides, wood preservatives, and colored gases and in printing and dyeing. Arsenic pentoxide dissolves and becomes liquid by absorbing moisture from the air (deliquescent) and is odorless.
Arsenic electron dot diagram
Arsenic triiodide can be precipitated from a hot solution of trivalent arsenic in hydrochloric acid by the addition of potassium iodide or it can be formed by treating elemental arsenic with a solution of iodine in carbon disulfide. Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. A step-by-step explanation of how to draw the AsF6- Lewis Dot Structure (Arsenic Hexafluoride ion).For the AsF6- structure use the periodic table to find the... A step-by-step explanation of how to draw the Lewis Structure for AsCl3 (Arsenic Trichloride).Get more chemistry help at http://www.thegeoexchange.org/chemis...
Arsenic electron dot diagram. Arsenic (As) is the least electronegative and goes at the center of the Lewis structure. Arsenic (As) is in Period Four on the periodic table and can hold more than 8 valence electrons. See the Big List of Lewis Structures. Transcript: This is the AsF5 Lewis structure. Arsenic has 5 valence electrons, Fluorine has 7, we have 5 Fluorines. The answer is B) trigonal pyramidal. To determine the molecular geometry of arsenic trichloride, AsCl_3, you must take a look at its Lewis structure. One arsenic trichloride molecule will have a total of 26 valence electrons - 5 from the arsenic atom and 7 from each of the three chlorine atoms. The arsenic atom will be bonded to the three chlorine atoms through single bonds that account for 6 ... Electron Dot Diagram for Halogens. 7. ... The nonmetals of the nitrogen family (nitrogen, phosphorous, arsenic) form ions with a charge of. 3-The nonmetals of the oxygen family (oxygen, sulfur, selenium) form ions with a charge of. 2-All of the elements of the alkali metals group form ions with a charge of. 1+ Modify. 2021-11-20. Create. 2005-03-27. Arsenic bromide appears as a yellowish white crystalline solid. Absorbs moisture from the air. Decomposed by water to form arsenic acid and hydrobromic acid, a corrosive. Intensely toxic. CAMEO Chemicals.
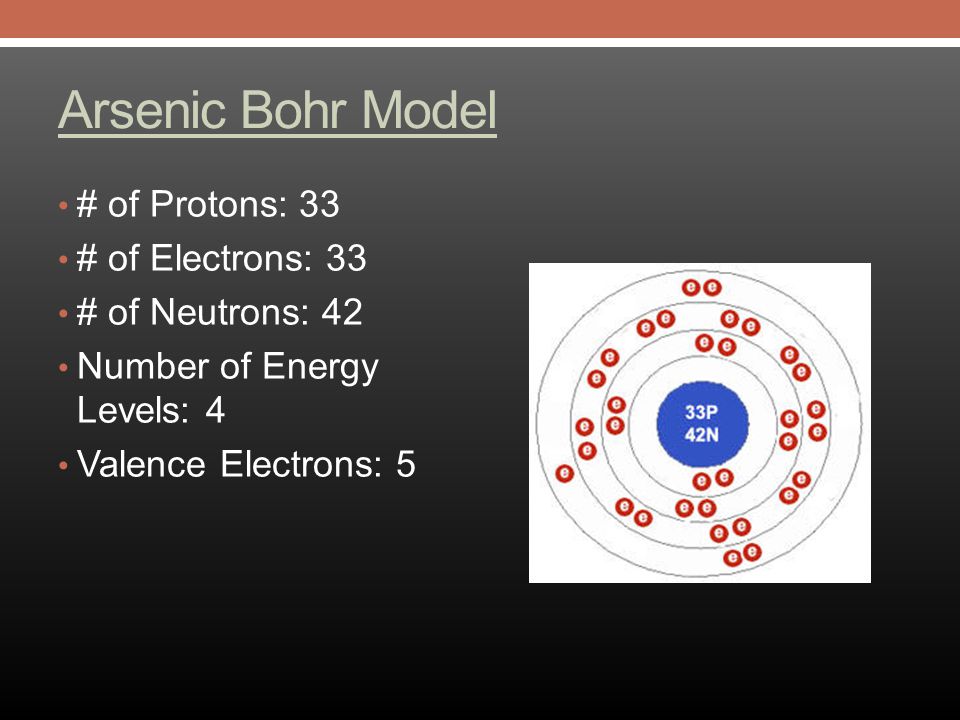
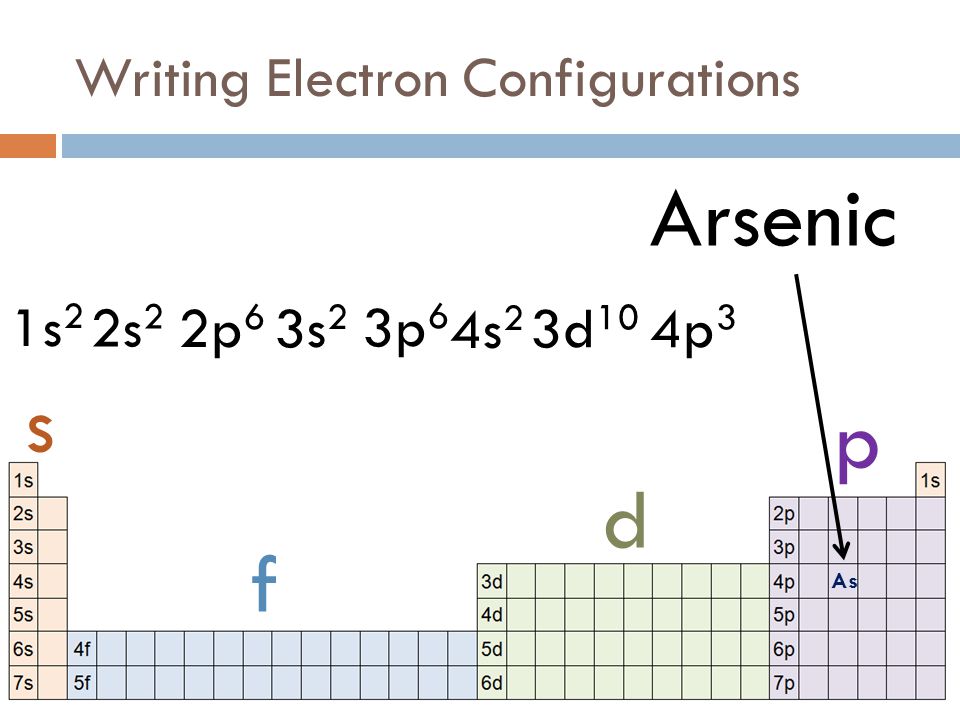
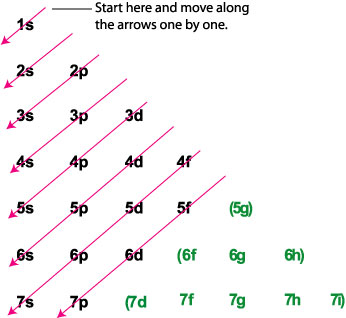
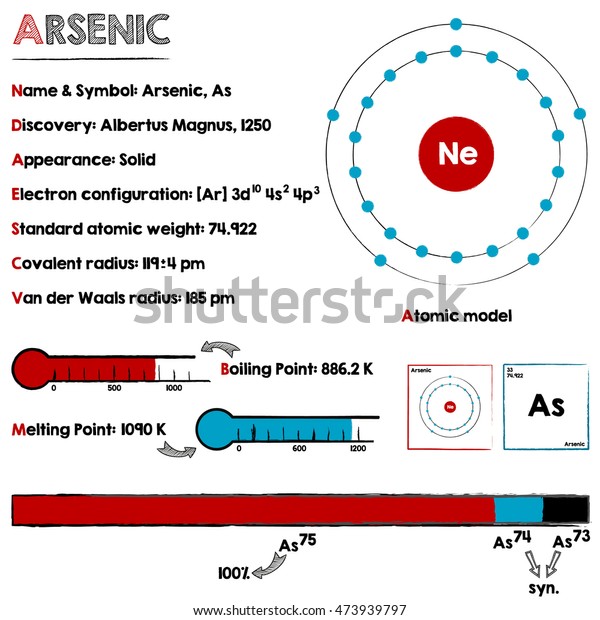
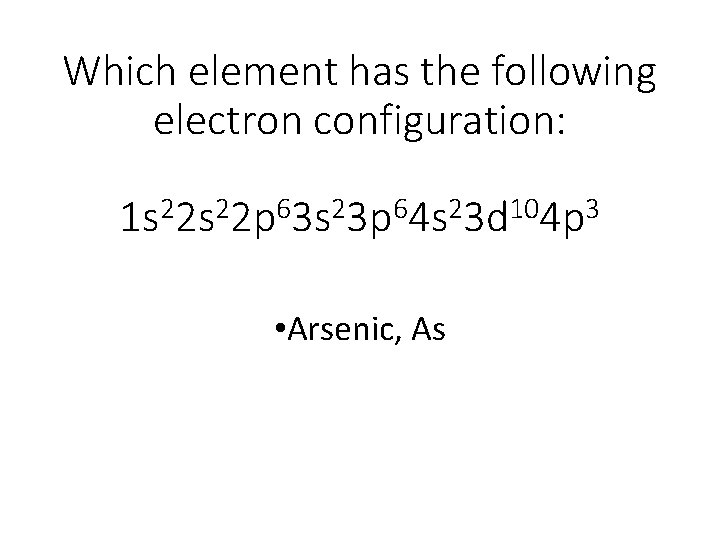
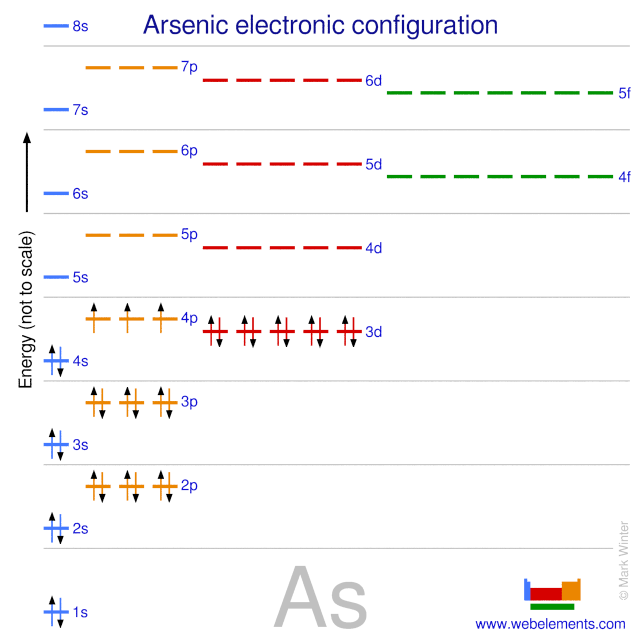
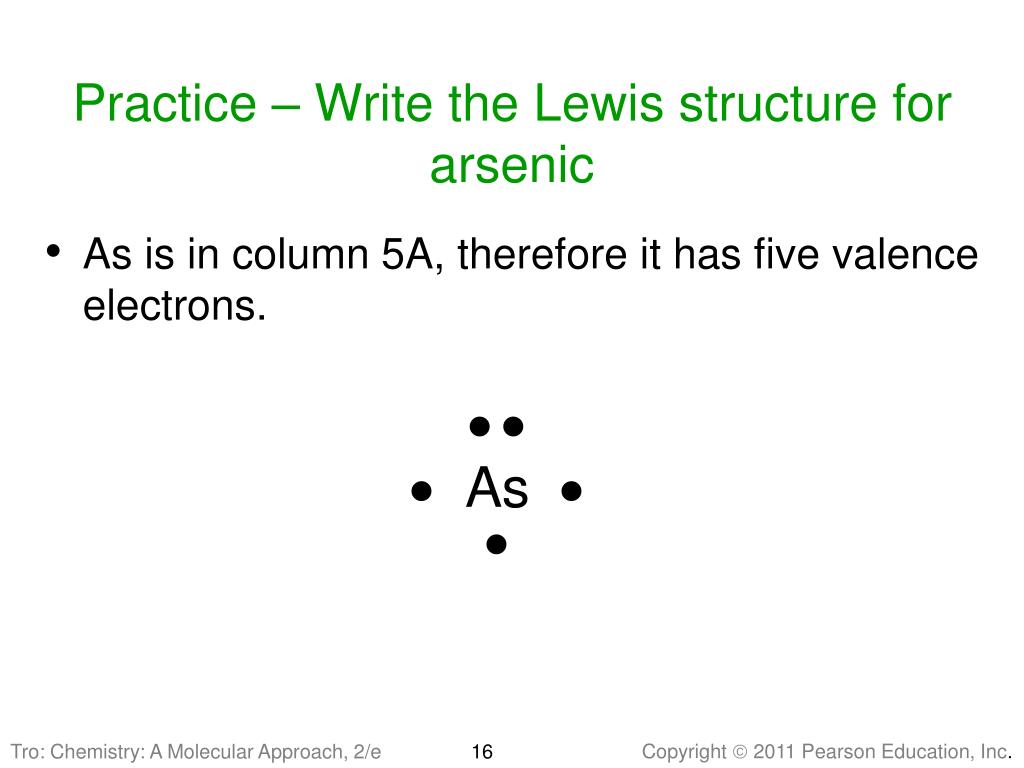
A step-by-step explanation of how to draw the Arsenic (As) Lewis Dot Structure.For the ArsenicLewis structure use the periodic table to find the total number... What is the electron dot diagram for arsenic? while just writing electronic configuration in shells (orbits) it is 2,8,18,3. While dividing into sub-shells it is 1s2, 2s2 2p6, 3s2 3p6, 4s2, 3d10, 4p3 Answers. Strontium (Sr) and Gallium (Ga) are the elements which have fewer than four dots in the electron dot diagrams. Explanation: Electron dot diagrams are the diagrams which represent the valence electrons in an element. The electrons are represented by the dots in these diagrams. Valence electrons in Arsenic (As) = 5. Electron dot diagrams, sometimes called Lewis dot diagrams, were first used by Gilbert N. Lewis in These diagrams are used as a. Comprehensive information for the element Arsenic - As is provided by this page including scores of Atomic Structure of Arsenic Electron Dot Model.
An electron-dot diagram is a graphical representation of the valence electrons of a certain element. The chemical symbol of an element placed in the middle and the valence electrons are represented by dots/5(7). Arsenic (As) has an atomic mass of Find out about its chemical and physical properties, states, energy, electrons, oxidation and more ... What is the electron dot notation for arsenic? After the 3d sublevel is filled, the remaining three electrons will be in orbitals in the 4p sublevel. Thus, the electron configuration for arsenic is [Ar]4s 2 3d 10 4p 3. Before writing the electron - dot structure for arsenic, note that arsenic's ten 3d electrons are not in the highest ... The Lewis structure for AsH 3 is similar to AsF 3 structure. The Arsenic atom goes in the center of the Lewis structure since it is the least electronegative atom. Remember that Hydrogen (H) atoms always go on the outside of a Lewis structure. For the AsH 3 Lewis structure there are a total of 8 valence electrons available. A step-by-step explanation of how to draw the AlN Lewis Dot Structure.For AlN we have an ionic compound and we need to take that into account when we draw th...
DRAWING LEWIS DOT DIAGRAMS 1) Given a Bohr model, count the 2 Mg DRAWING ELECTRON DOT DIAGRAMS Draw the Dot Diagram for arsenic (As). Comprehensive information for the element Arsenic - As is provided by this page including scores of Atomic Structure of Arsenic Electron Dot Model. The left diagram shows a Lewis dot structure of sodium with.
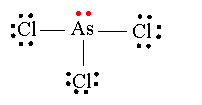
The Lewis structure for AsCl 3 is similar to AsF 3 structure. Since they are in the same Group on the periodic table they each have the same number of electrons their structures are similar. The Arsenic atom goes in the center of the Lewis structure since it is the least electronegative atom.
Arsenic trifluoride is included on this list. Clean Air Act as amended in 1990, Sect. 112 (b) (1) Public Law 101-549 Nov. 15, 1990 Hazardous Substances Data Bank (HSDB)
A step-by-step explanation of how to draw the AsBr3 Lewis Dot Structure.For the AsBr3 structure use the periodic table to find the total number of valence el...
Arsenic is isoelectronic with nitrogen (they are both Group V elements), so there are 5 valence electrons.A handy way to illustrate these valence electrons is to use Lewis diagrams, also called electron dot diagrams. These diagrams show the symbol of the element with as many dots around it as there are electrons in the outermost energy level.
Arsenic acid is an arsenic oxoacid comprising one oxo group and three hydroxy groups attached to a central arsenic atom. It has a role as an Escherichia coli metabolite. It is a conjugate acid of an arsenate (1-) and an arsenate ion. Arsenic acid, liquid appears as a clear colorless aqueous solution. Noncombustible.
arsenic (As) The electron dot diagram shows the arrangement of dots without identifying the element. Which element's symbol could replace the question mark in the diagram? tellurium (Te) Helium (He) and neon (Ne) are elements in Group 8A of the periodic table. How do the electron dot diagrams of these elements compare?
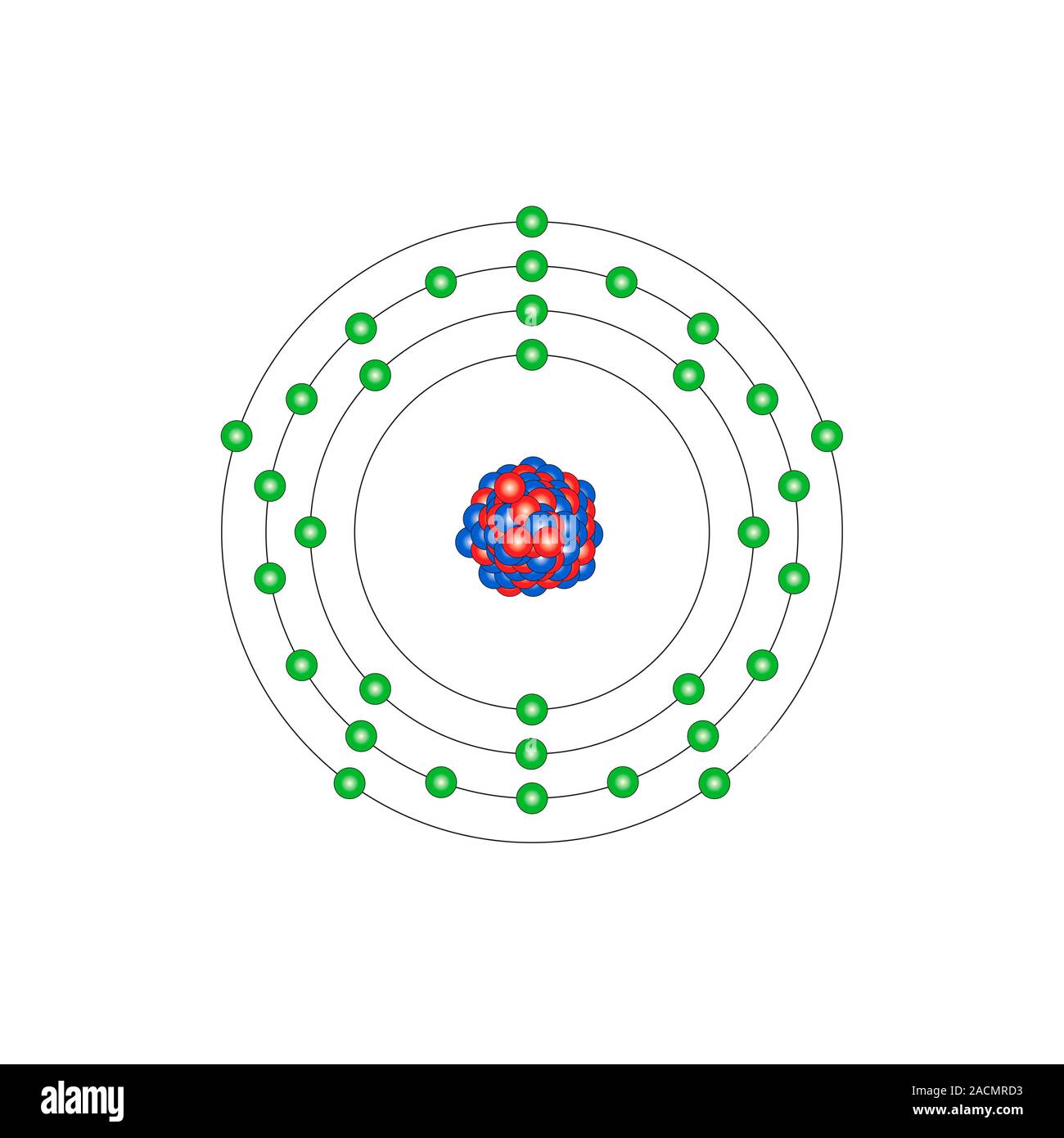
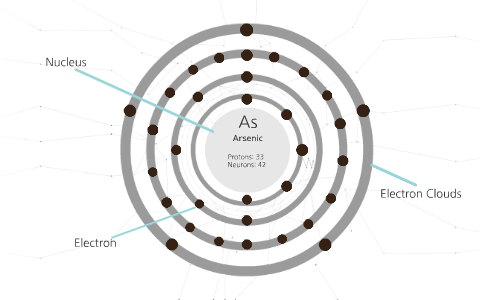
Arsenic is a metalloid with the atomic number 33 which means it has 33 protons in its nucleus and 33 electrons in its electron cloud. Arsenic is a carcinogen, associated with lung cancer when inhaled. It is mainly produced as a by-product of refining certain sulphide ores. It is naturally occurring in many household products.
What is the Lewis dot structure for arsenic? Chemical Bonding: AsH3 Lewis Structure The Arsenic atom goes in the center of the Lewis structure since it is the least electronegative atom. Remember that Hydrogen (H) atoms always go on the outside of a Lewis structure. For the AsH3 Lewis structure there are a total of 8 valence electrons available.
Therefore, the total number of valence electrons in Arsenic Trifluoride [AsF 3] is given by: 5[As] + 21[F] = 21 valence electrons. AsF3 Lewis Structure. As you already know, the Lewis structure of a molecule or compound helps give insight into various molecular properties.
The Lewis structure for AsF 3 is similar to AsCl 3 structure. Since they are in the same Group on the periodic table they each have the same number of electrons their structures are similar. The Arsenic atom goes in the center of the Lewis structure since it is the least electronegative atom.
A step-by-step explanation of how to draw the Lewis Structure for AsCl3 (Arsenic Trichloride).Get more chemistry help at http://www.thegeoexchange.org/chemis...
A step-by-step explanation of how to draw the AsF6- Lewis Dot Structure (Arsenic Hexafluoride ion).For the AsF6- structure use the periodic table to find the...
Arsenic triiodide can be precipitated from a hot solution of trivalent arsenic in hydrochloric acid by the addition of potassium iodide or it can be formed by treating elemental arsenic with a solution of iodine in carbon disulfide. Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26.

























0 Response to "44 arsenic electron dot diagram"
Post a Comment