45 free energy diagram enzyme
by TP Silverstein · 2021 · Cited by 1 — In at least two papers published in this Journal, enzyme free energy diagrams were presented showing enzyme–substrate binding to be ...Abstract · Author Information Each step is catalyzed by a specific enzyme Enzyme 1 A B Reaction 1 Enzyme 2 C Reaction 2 Enzyme 3 D ... release of free energy and is spontaneous An endergonic reaction absorbs free energy from its surroundings and is nonspontaneous Reactants Energy Products Progress of the reaction
Download scientific diagram | Comparing the free energy surfaces for an enzyme reaction and the corresponding reaction in solution.
Free energy diagram enzyme
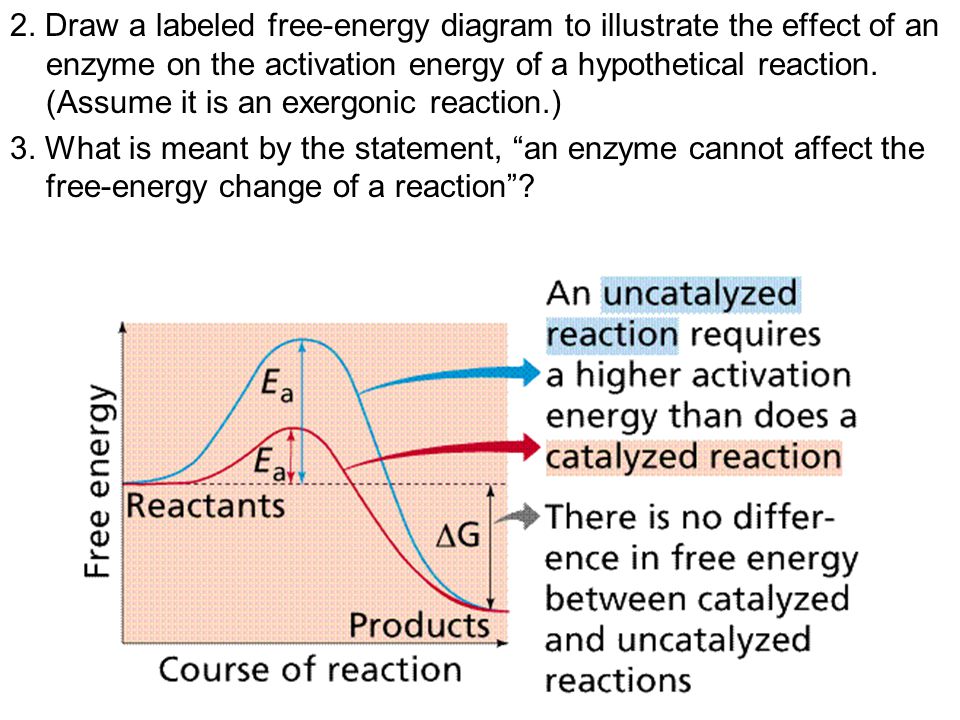
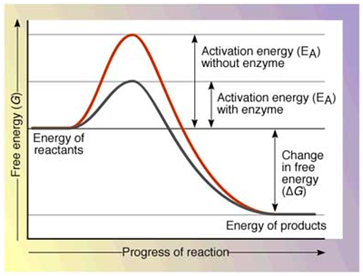
Textbook treatments In many books,2-8 a very simple general energy diagram is depicted (Fig 1) which, in addition to being inaccurate in the case of enzyme catalysis, can lead to a misunder- standing of the mechanism of the enzymic action. These textbooks properly emphasize the fact that the actual free energy difference between reactants and ... Gibbs free energy reaction coordinate profiles found in some textbooks. The energy diagram for a reaction model consisting of one enzyme, one substrate, and one product is depicted in many books where it is compared with that for the uncatalyzed reaction. Enzyme Energy Diagram. energy diagrams for enzyme catalysed reactions a you have free access to this content biochemical education volume 25 issue 2 version of record online 30 jun 2010 structural biochemistry enzyme gibbs free energy graph the gibbs free energy graph shows whether or not a reaction is spontaneous whether it is exergonic or endergonic Δg is the change in free energy
Free energy diagram enzyme. In at least two papers published in this "Journal," enzyme free energy diagrams were presented showing enzyme--substrate binding to be nonspontaneous. I present here several different approaches showing that enzyme--substrate binding is "always" spontaneous, and the y-axis of the reaction progress diagrams presented previously is in fact standard enthalpy (H°), and not free energy. Energy diagram of enzyme-catalyzed and uncatalyzed reactions, from Wikipedia The peak of this energy diagram represents the transition state: an intermediate stage in the reaction from which the reaction can go in either direction. Enzyme and Energy In a metabolic pathway, a series of enzymatic reactions catalyzes the conversion of molecule A to molecule E. Several intermediate steps are involved in which the product of one reaction becomes the substrate for the next. The graph illustrates the changes of free energy that occur at each step in the pathway. today we're going to talk about how enzymes can influence reactions activation energy but first let's review the idea that enzymes make biochemical reactions go faster and in order to do that they use a bunch of different catalytic strategies now there are lots of different catalytic strategies that enzymes use but a couple of the key ones are acid-base catalysis where enzymes use their acidic ...
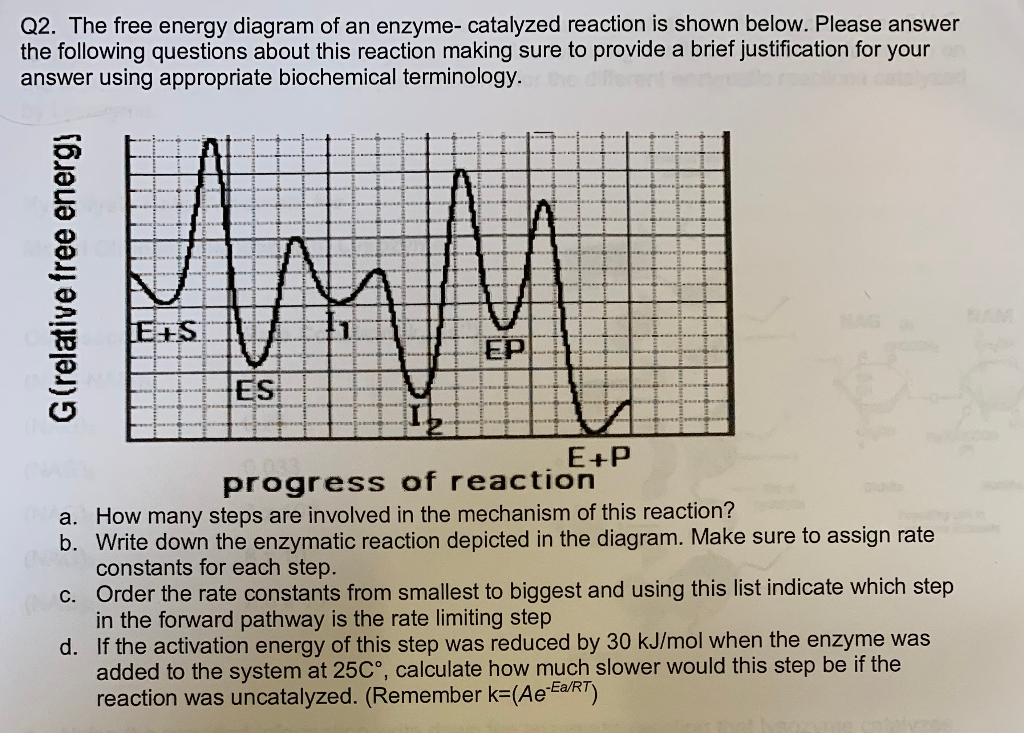
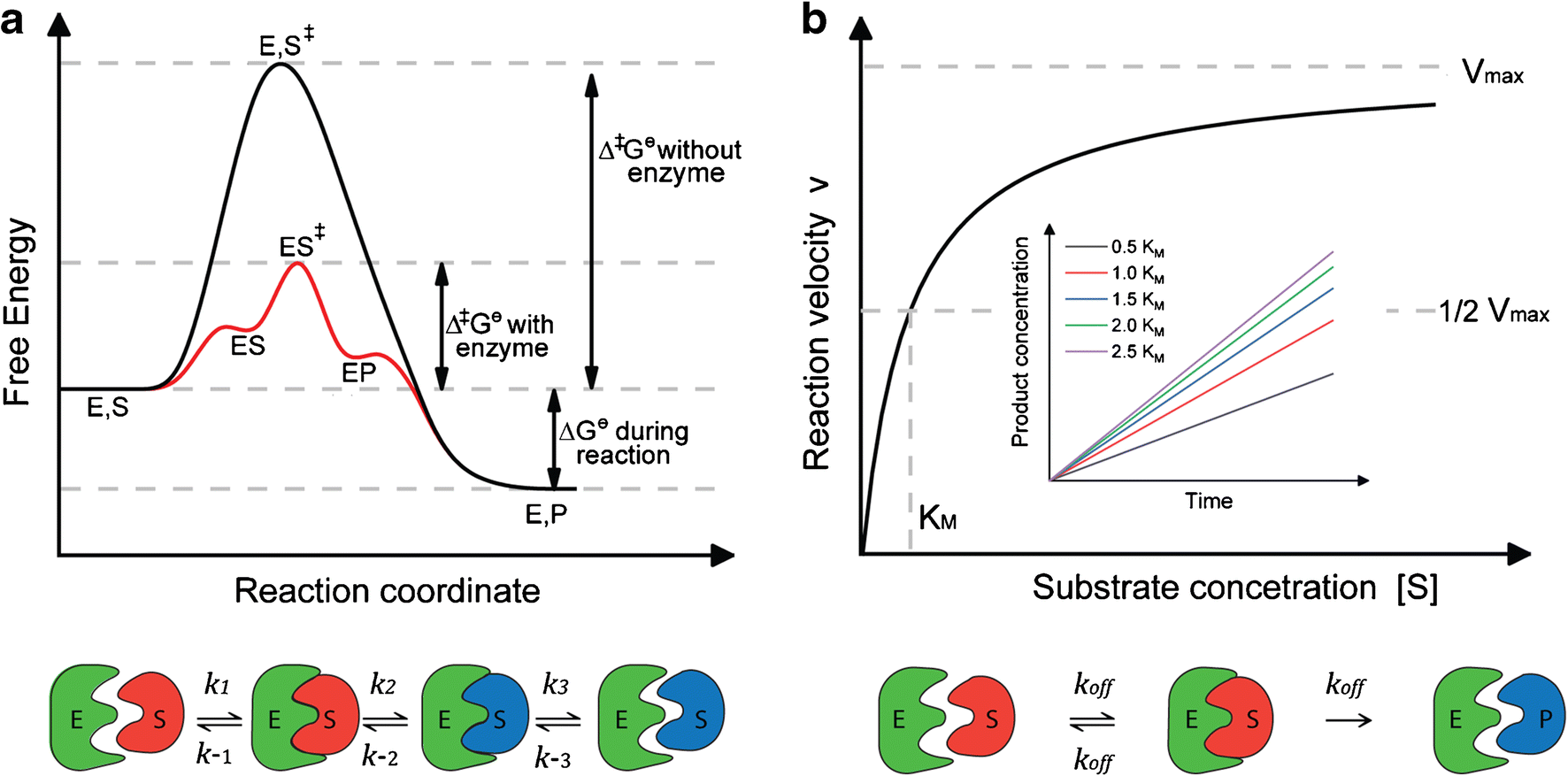
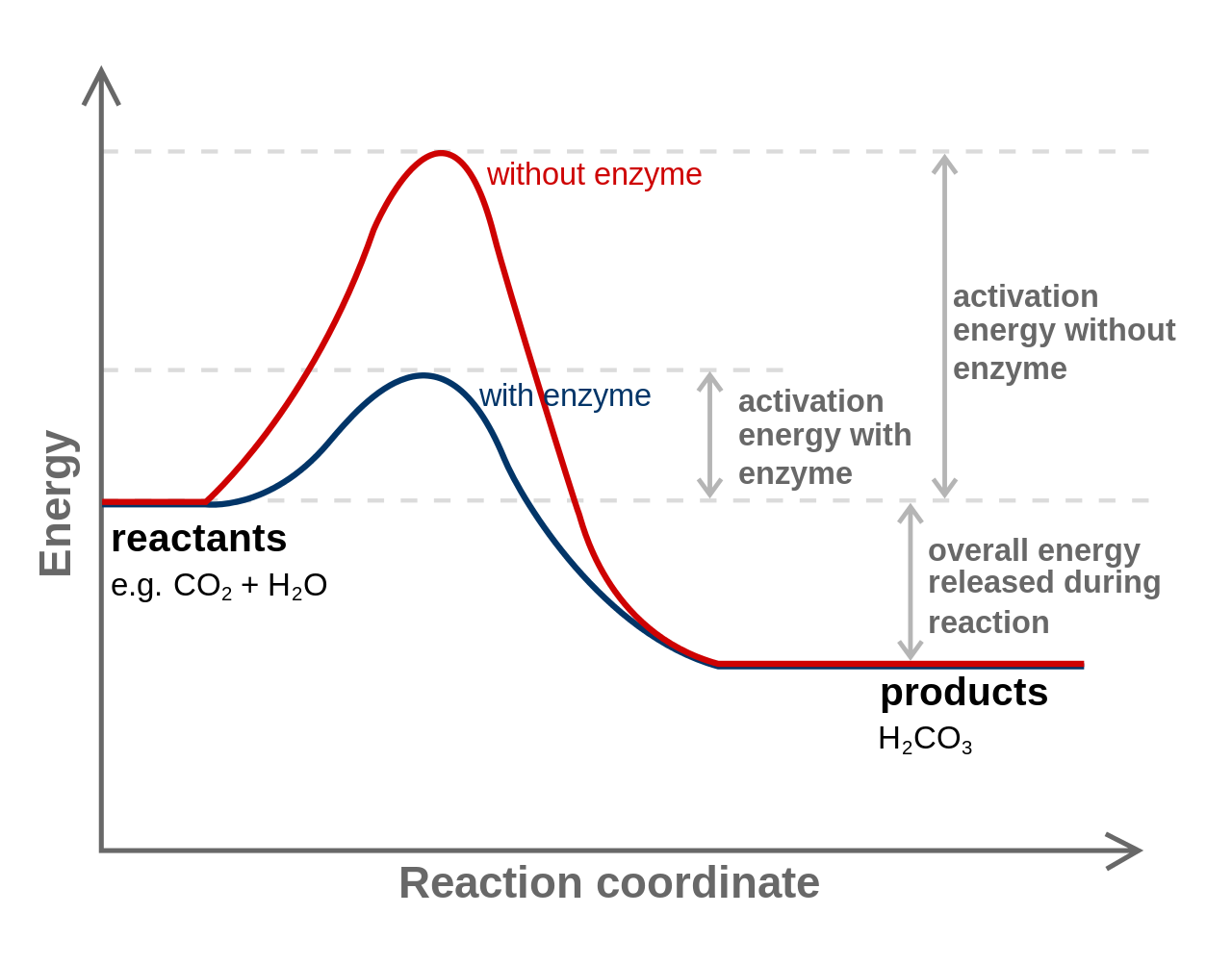
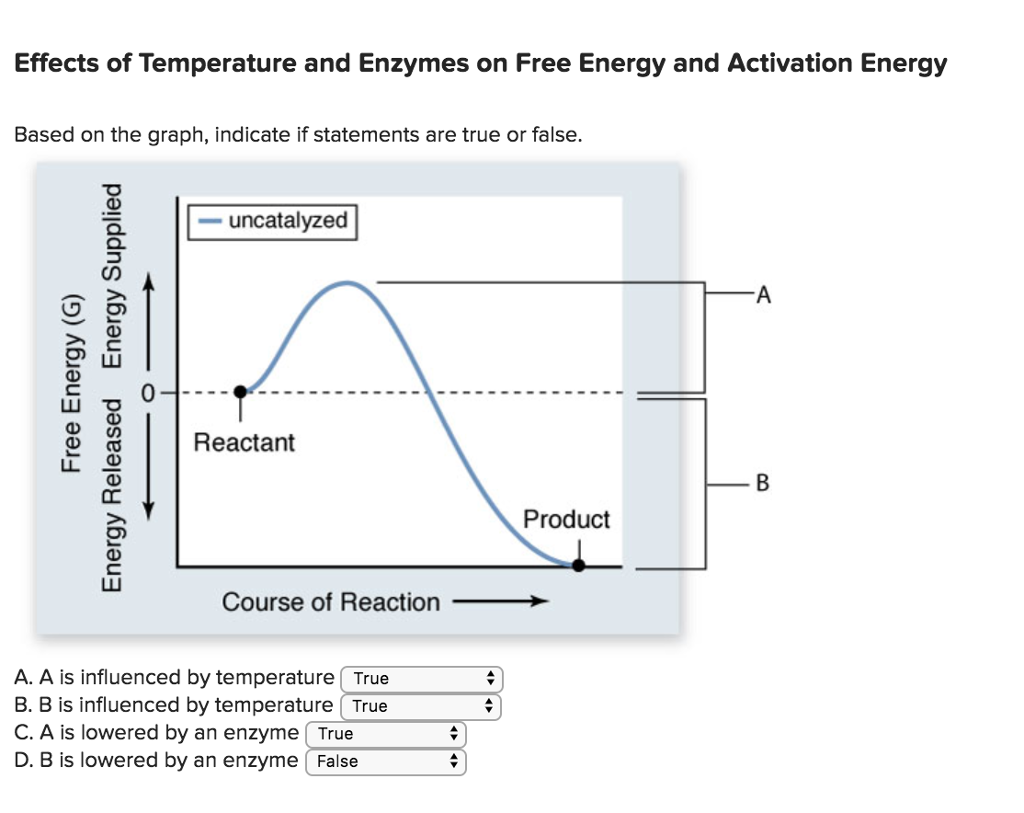
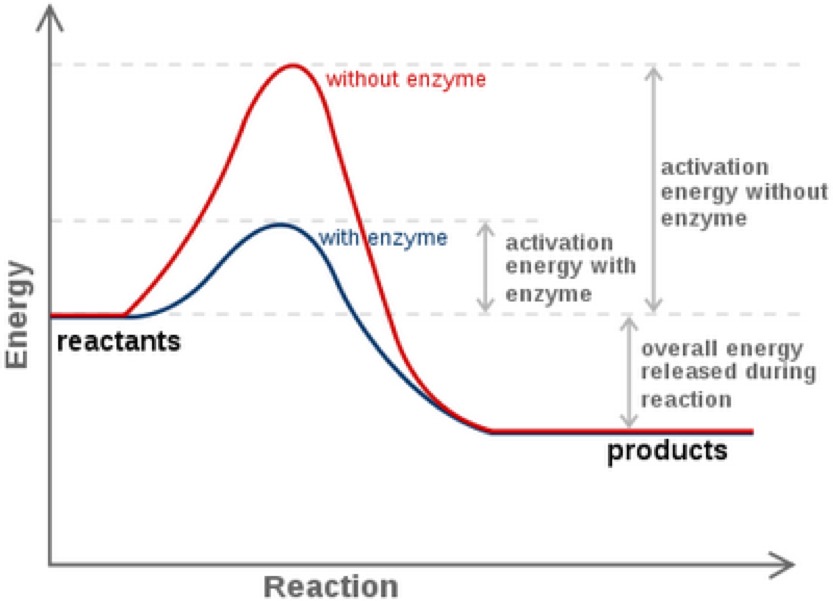
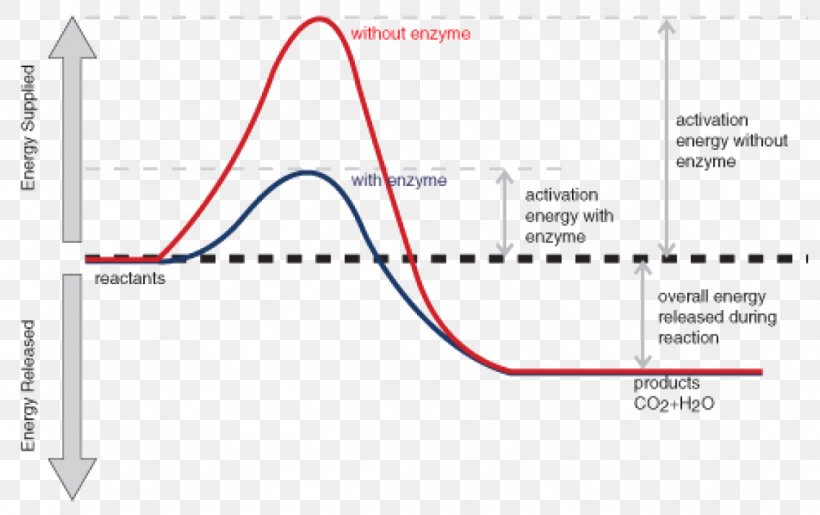
Enzymes have high substrate specificity, and can even show regiospecificity that leads to the generation of stereospecific products. Figure 7.1 Effect of an enzyme on reducint the activation energy required to start a reaction where (a) is uncatalyzed and (b) is an enzyme-catalyzed reaction. Figure from Peter K. Robinson. The free energy released from the exergonic reaction is absorbed by the endergonic reaction. One example of energy coupling using ATP involves a transmembrane ion pump that is extremely important for cellular function. Free Energy Diagrams. Free energy diagrams illustrate the energy profiles for a given reaction. Enzymes decrease the Gibbs free energy of activation, but they have no effect on the free energy of reaction. Enzymes work by lowering the activation energy (E_a or ΔG^ ) for a reaction. This increases the reaction rate. Free energy of reaction = free energy of product - free energy of substrate The free energy of the product is the same as it would be without the enzyme. Thus, the enzyme ... addition, enzymes also promote catalysis by positioning key acidic or basic groups and metal ions in the right position for catalysis. In reality, the free energy diagram for an enzyme-catalyzed reaction is considerably more complicated than the exam-ple in Fig. 4.2. Typically an enzyme-catalyzed reaction will involve multiple steps,
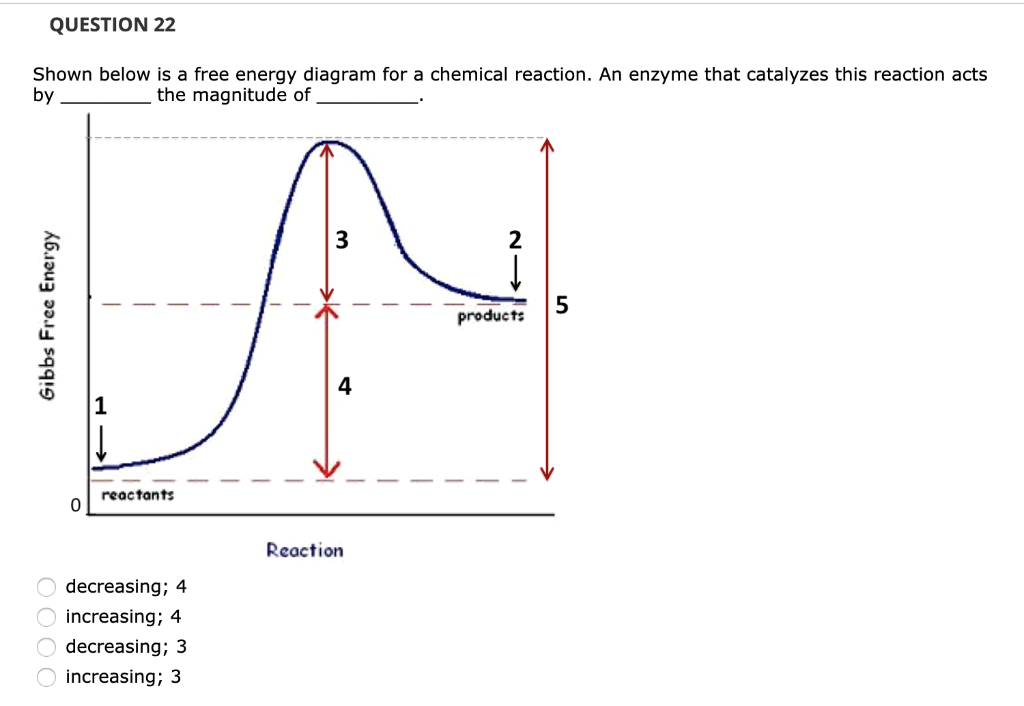
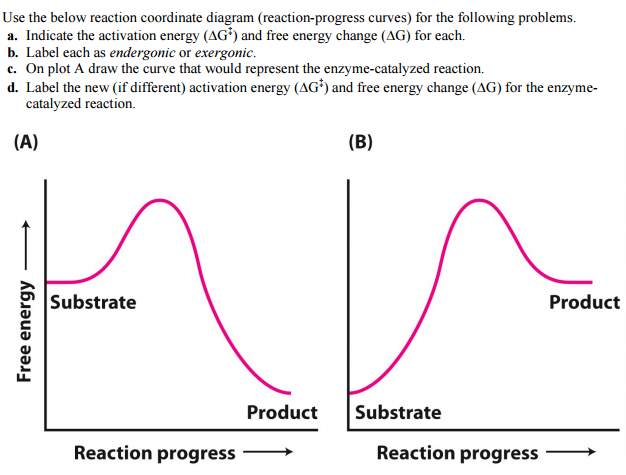
Gibbs free energy reaction coordinate profiles found in some textbooks. The energy diagram for a reaction model consisting of one enzyme, one substrate, and one product is depicted in many books where it is compared with that for the uncatalyzed reaction. Figure 4.7 Enzymes lower the activation energy of the reaction but do not change the free energy of the reaction. The chemical reactants to which an enzyme binds are called the enzyme's substrates. Reaction coordinate diagrams for exergonic and endergonic reactions. In the exergonic reaction, the reactants are at a higher free energy level than the products (reaction goes energetically downhill). In the endergonic reaction reaction, the reactants are at a lower free energy level than the products (reaction goes energetically uphill). The graph below is a free energy diagram of the forward reaction Y + X + Z without an enzyme present. Free Energy M X+Z Reaction Progress Which of the following is true for the value labelled "a" in the figure if an enzyme were added to the reaction? The value for "a" would increase. The value for "a" would not change. The value for "a" would ...
• Electrons drop in free energy as they go down the chain and are finally passed to O 2, forming H 2O NADH FADH 2 2 H+ + 1/ 2 O 2 0 2 e-2 e-2 e-H 2O NAD+ Multiprotein complexes (originally from NADH or FADH 2) I II III IV 50 40 30 20 Free energy ( 10 G O 2) FMN Fe•S Fe•S FAD Q Cyt b Cyt c 1 Cyt c Cyt a Cyt a 3 Fe•S
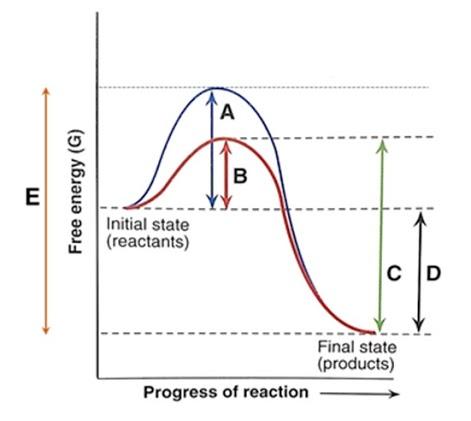
Consider the transition state diagram of (a) a nonenzymatic reaction and the corresponding enzyme-catalyzed reaction in which (b) S binds loosely to the enzyme and (c) S binds very tightly to the enzyme. ... Enzymes lower the free energy change for the reactions they catalyze. Enzymes catalyze reactions at their active site.
•Enzyme decreases Gibbs Free Energy of Activation; •Enzyme stabilizes Transition state of a chemical reaction; •Enzyme does not alter the energy levels of substrates and products; •Enzyme increases the rate at which reaction occurs, but has no effect on overall change in energy of the reaction; •Enzyme increases the rate of chemical ...
Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction. Label the energy diagram and answer the question that follows% (1). Catalyzed reactions have a lower activation energy (rate-limiting free energy of activation) than the corresponding uncatalyzed reaction, resulting in a higher ...
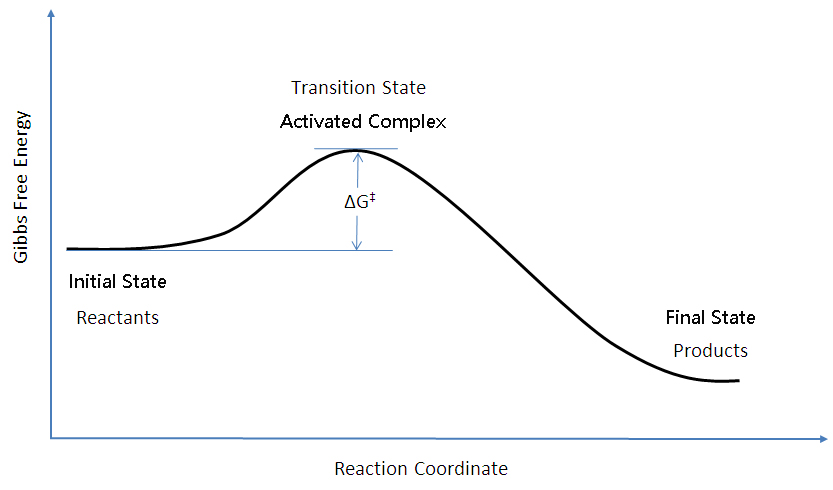
Left: Reaction coordinate diagram for an enzyme-catalyzed reaction representing the mechanistic model we are considering. The free energy of activation, Δ G ‡, which for our present purposes can be considered as equivalent to activation energy, is much lower for the catalyzed reaction compared with the uncatalyzed reaction:
Explore the role of enzymes in making a reaction more likely to happen quickly. By Ross Firestone. Created by Ross Firestone.Watch the next lesson: https://w...
Illustrating Enzyme Inhibition Using Gibbs Energy Profiles. Journal of Chemical Education 2012 , 89 (6) , 732-737. DOI: 10.1021/ed200395n.
The free energy graph can be used to determine whether the reaction will be spontaneous or not by evaluating the ΔG. ΔG can be found by subtracting the free ...
ATP— The Free Energy Carrier 5. Extension Questions. 13. Describe or draw a diagram of adenosine monophosphate (AMP). 14. If ADP were to be hydrolyzed in a similar manner to ATP, would you expect the reaction to be
Introduction. Free energy diagrams are undoubtedly one of the most useful and fundamental ways to illustrate and explain enzyme catalysis, and essentially any biochemistry textbook uses profiles of free energy and reaction coordinates to introduce enzyme function.
Catalysts, and among them enzymes which are biological catalysts, act by considerably decreasing the free energy of activation. The smaller the quantity of free energy of activation to be supplied, the more effective the catalyst (see fig. 2-1). The main properties of a catalyst may be briefly summarised as follows: 1.
Jun 2, 2019 — How do enzymes reduce Ea? Enzyme Mechanisms. Kinetics of an un-catalyzed chemical reaction vs. a catalyzed chemical reaction. Gibbs Free Energy ...
by JC Aledo · 2003 · Cited by 19 — For any chemical reaction, regardless of how negative the actual change of Gibbs free energy (ΔG) is, the reactants must overcome a kinetic ...Abstract · DIFFERENT BOOKS... · ENERGY DIAGRAMS FOR... · CONCLUSIONS
The energy used to break the bonds in the reactants so they can be reformed in the products is called the energy of activation. As the hydrogen gas and oxygen gas bonds are broken and new ones are formed the system has a net loss of free energy. In a system, such as the Hindenburg, that energy is released as heat and light.
Watch more videos on http://www.brightstorm.com/science/chemistrySUBSCRIBE FOR All OUR VIDEOS!https://www.youtube.com/subscription_center?add_user=brightstor...
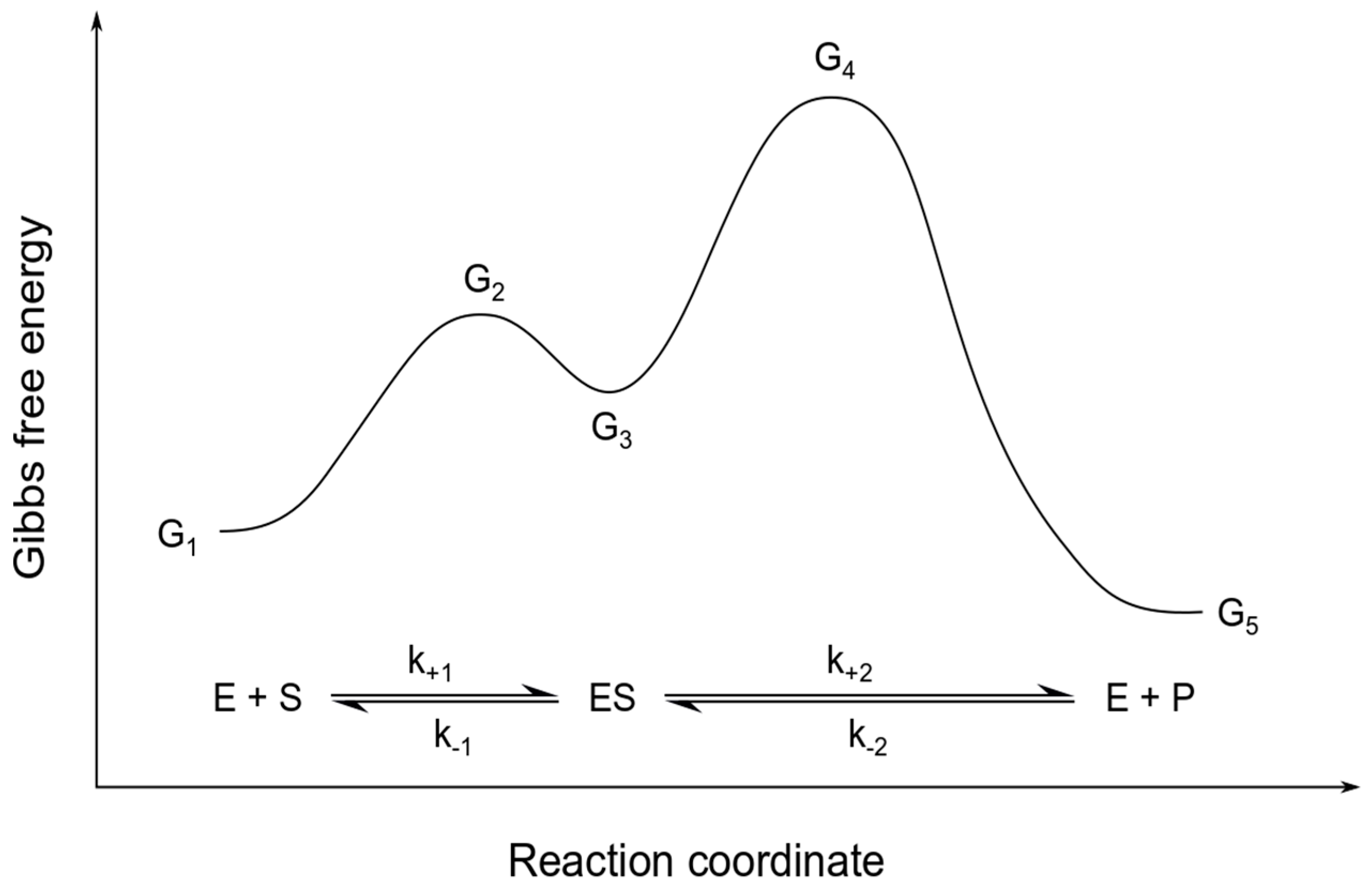
Biology questions and answers. Part 1: Free Energy Profiles 1. Given a set of rate constants for an enzymatic reaction and the knowledge of transition state theory you should be able to construct a free energy profile/diagram for the reaction. The following enzyme (E) converts substrate (S) to product (P) via one chemical intermediate (t).
Enzyme Energy Diagram. energy diagrams for enzyme catalysed reactions a you have free access to this content biochemical education volume 25 issue 2 version of record online 30 jun 2010 structural biochemistry enzyme gibbs free energy graph the gibbs free energy graph shows whether or not a reaction is spontaneous whether it is exergonic or endergonic Δg is the change in free energy
Gibbs free energy reaction coordinate profiles found in some textbooks. The energy diagram for a reaction model consisting of one enzyme, one substrate, and one product is depicted in many books where it is compared with that for the uncatalyzed reaction.
Textbook treatments In many books,2-8 a very simple general energy diagram is depicted (Fig 1) which, in addition to being inaccurate in the case of enzyme catalysis, can lead to a misunder- standing of the mechanism of the enzymic action. These textbooks properly emphasize the fact that the actual free energy difference between reactants and ...
Entropy Free Full Text Chemical Reaction Networks Possess Intrinsic Temperature Dependent Functionality Html


























0 Response to "45 free energy diagram enzyme"
Post a Comment