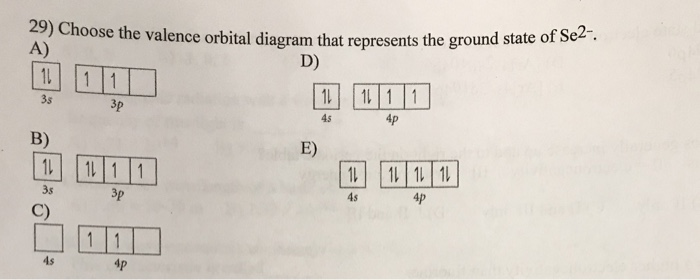
40 choose the valence orbital diagram that represents the ground state of se2-.
Ground State Electron Configuration For Nitrogen. When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s 2 2s 2 2p 3.Below you can get the full image representation which will help you to understand the topic more easily. Chemistry questions and answers. 5. Choose the valence orbital diagram that represents the ground state of Se2- 3s b. 3p 3s 3p 4p d. 4s 4p 4p 6. Place the following in order of decreasing IE1 4s Cs Mg Ar a. Ar > Mg> Cs b. Mg> Ar > Cs c. Cs > Mg>Ar d. Cs > Ar> Mg e. Mg > Cs> Ar 7. Place the following in order of increasing IE1.
The electron configuration of a neutral zinc atom is 1s22s22p63s23p63d104s2. The Zn2+ ion has lost two electrons, which leaves it with 30 protons and 28 electrons. The electron configuration of Zn2+ is 1s22s22p63s23p63d10. Zinc is a d-block element, also known as a transition element. For the d-block elements, the outermost s-sublevel has ...

Choose the valence orbital diagram that represents the ground state of se2-.
Example: 1s 2 For writing ground state electron configurations, a few main steps should be followed. Find the amount of electrons in the atom. Example: Na: 11 e - Na +: 10 e -. Fill orbitals following the model until all electrons have been accounted for. Example: Na: 11 e - 1s 2 2s 2 2p 6 3s 1 or Na +: 1s 2 2s 2 2p 6. Study Chem 170 Chapter 8 flashcards from Brendan Jackson's class online, or in Brainscape's iPhone or Android app. Learn faster with spaced repetition. Choose The Valence Orbital Diagram That Represents The Ground State Of Zn. The partial orbital diagram below where n could be any valid quantum number. 11 years aieee chapterwise by mtg hope it helps by shreesha rao 2 in types school work the d block elements lardbucket alloys and pounds of the d block elements are important ponents of the ...
Choose the valence orbital diagram that represents the ground state of se2-.. Choose the orbital diagram that represents the ground state of N. 11 11 2s 1s 2p... Choose the orbital diagram that represents the ground state of N. 11 11 2s 1s 2p 11 11 2P 1. 11 2r 25 O 11 Is O 11111 2p 1s 25 O 1. 11 15 2p 1 1 1 15 23 20 Submit Request Answer 17. Write ground state electron configuration AND Orbital Box Diagram for silicon. Question 2 05 out of 05 points choose the statement that is true. Choose the orbital diagram that represents the ground state of n. Orbital diagram that represents the ground state of n 1s2 2s2 2p3 give the set of four quantum numbers that could represent the last electron added using the aufbau principle to the cl atom. 0 Comments. on Orbital Diagram For Germanium. orbital. Because an electron can have either one of two spins, any orbital can hold a maximum of four . The orbital diagram for germanium is. 1s. 2s. 2p. 3s. Oxidation States, +4,2. Electrons Per Shell, 2 8 18 4. Electron Configuration, [Ar] 3d10 4s2 4p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2. Choose the orbital diagram that represents the ground state of N. Choose the valence orbital diagram that represents the ground state of Zn. ... Give the ground state electron configuration for Se2. [Ar]4s23d104p6. Choose the ground state electron configuration for Zn2. [Ar]3d10.
Choose the valence orbital diagram that represents the ground state of Se 2 A B from CHM 2045 at University of South Florida. Choose the valence orbital diagram that represents the ground state of Se 2−. We’re being asked to construct the orbital diagram for Se2–. Step 1: Determine the electron configuration of the neutral element. Step 2: Determine the electron configuration of the ion. Step 3: Construct the orbital diagram for the ion. Valence Bond Model vs. Molecular Orbital Theory . Because arguments based on atomic orbitals focus on the bonds formed between valence electrons on an atom, they are often said to involve a valence-bond theory.. The valence-bond model can't adequately explain the fact that some molecules contains two equivalent bonds with a bond order between that of a single bond and a double bond. The above diagram roughly depicts the relative energy difference between these three ways of filling 2 electrons into the three p orbitals. Ground State: 1s22s22p x 12p y 1 (or 1s22s22p x 12p z 1 or 1s22s22p y 12p z 1) [CAUTION: these don't explicitly state the electron's spin!]
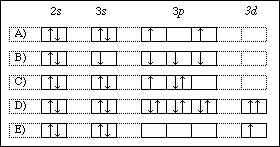
Chapter 8 Periodic Properties of the Elements. 1) Give the ground state electron configuration for Se. 2) Give the ground state electron configuration for I. 3) Give the ground state electron configuration for Sr. 4) Give the ground state electron configuration for Pb. 5) Give the ground state electron configuration for Cd. Which ground-state ion does not have an electron configuration described by the following orbital diagram? asked Aug 24, 2019 in Chemistry by Parreira general-chemistry This preview shows page 14 - 17 out of 24 pages. -:-) Choose the orbital diagram that represents the ground state of N. A) . DIJ · DIJ I 1 I 1 I 1 I ls 2.s 2p B) DIJ OIJ lJlJIIJ Is 2s . 2p C) ITJ DJ 11~ 11~ I 1 I ls 2s 2p l.s 2s 2p E) DI] DI] 11~ I H [ill ls 2s 2p --) The element that conesponds .to the electron configuration ls22 ... Choose the valence orbital diagram that represents the ground state of zn. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 explanation. 8 2 Hybrid Atomic Orbitals Chemistry Chemistry electron configuration electron configuration. Choose the valence orbital diagram that represents the ground state of zn. 11 years aieee chapterwise by mtg hope it helps by shreesha ...
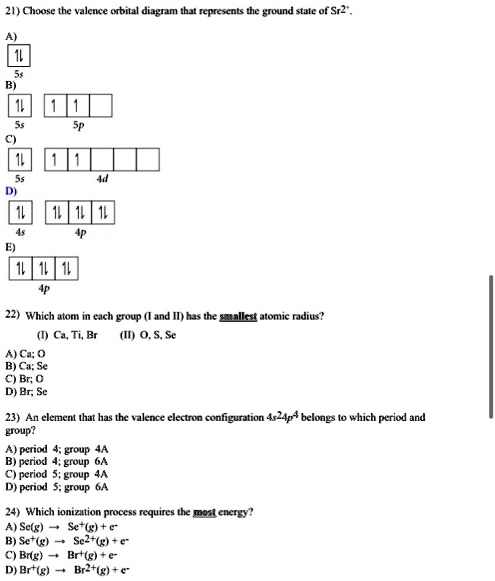
Strontium is atomic number 38 so its electron configuration can be written: 2.8.18.8.2. In orbital notation this is: 1s22s22p63s23p63d104s24p65s2. The noble gas shorthand notation is: [Kr]5s2. When strontium forms a 2+ ion the 2 outer 5s electrons are lost so Sr2+ can be written as: 1s22s22p63s23p63d104s24p6.
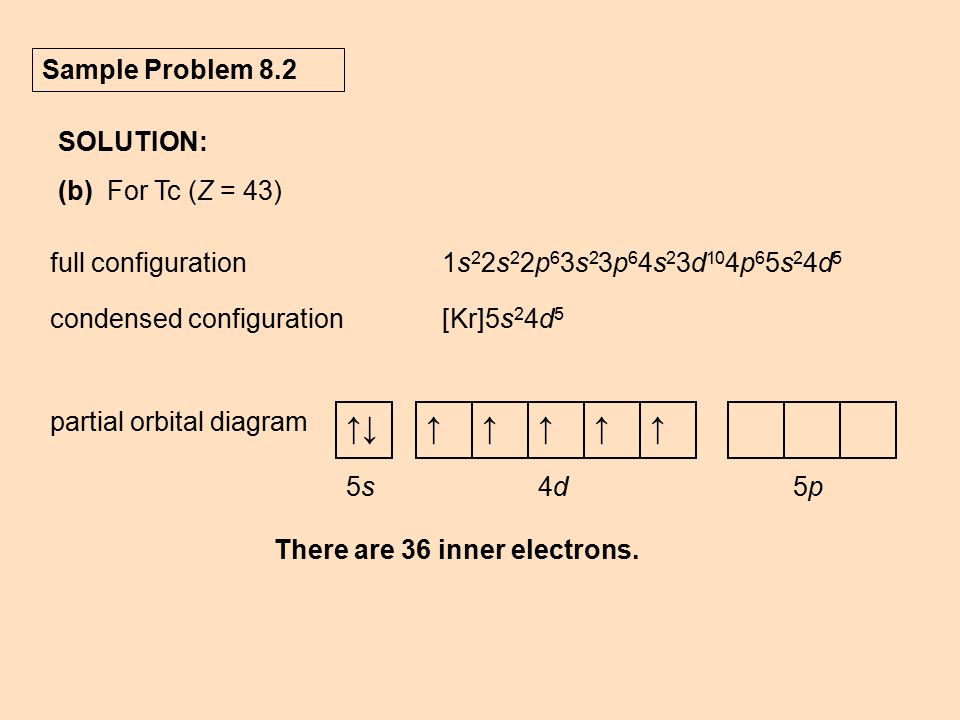
So here we have the electron configuration right now, five S. Fields before 40. So that first in the orbital diagram, we have one electron here. Normally, we have to. But the energy difference is so minimal between the five S. And the 40 that the electron can jump up, um and that would be slightly more stable. And then in the D sub level, the electrons don't pair up until they have to.
Choose the valence orbital diagram that represents the ground state of se2. Choose the paramagnetic species from below. 5 a it i1 i el 55 46 b if m at 71 45 4p 6 write out the orbital diagram that represents the ground state of as. Choose the valence orbital diagram that represents the ground state of se2.
Choose the valence orbital filling diagram that best represents the ground state of Br -. Learn this topic by watching The Electron Configuration: Ions Concept Videos All Chemistry Practice Problems The Electron Configuration: Ions Practice Problems
Exam 4 Review: Ch.8-9. Electromagnetic radiation with a wavelength of 745 nm appears as red light to the human eye. The energy of one photon of this light is ________ J. Calculate the wavelength (in nm) of the blue light emitted by a mercury lamp with a frequency of 6.19 × 10^14 Hz. Nice work!
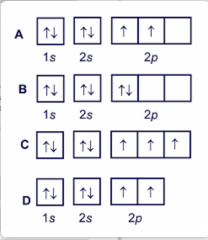
12) 12) Choose the valence orbital diagram that represents the ground state of Zn. A) 1L 1L 1L 1L 1L 4s 3d B) 16 11 111 1s 2s 2p C) 11 1L 1L 1L 1 1 4s 3d D) 11 1L 1L 1L 1L 1L 3d E) 11 4s 13) 13) Which of the following elements is classified as a metalloid? B) calcium C) uranium A) gold D) fluorine E) antimony Answer
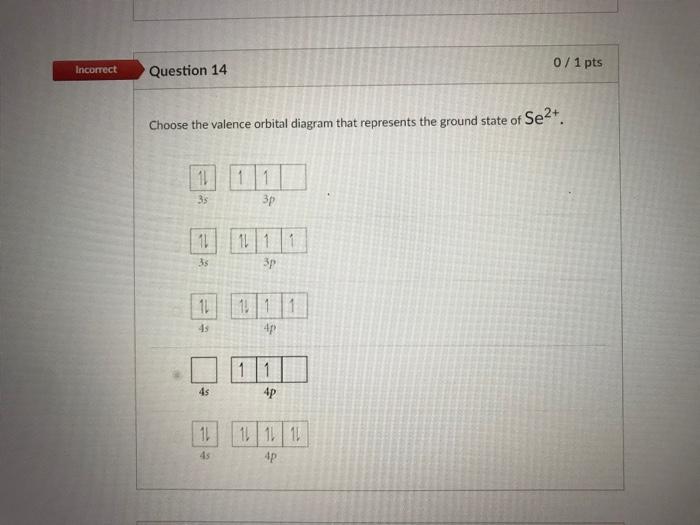
D) E) Answer: E 15) Write out the orbital diagram that represents the ground state of As. How many unpaired electrons are there? A) 0 B) 4 C) 3 D) 2 E) 1 Answer : C
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of .. Rb+, Se2−. The first orbital (an s orbital) can contain only two electrons.. Rubidium.
Orbital diagram that represents the ground state of n 1s2 2s2 2p3 give the set of four quantum numbers that could represent the last electron added using the aufbau principle to the cl atom. Question 3 05 out of 05 points choose the valence orbital diagram that represents the si. Question 2 05 out of 05 points choose the statement that is true.
Choose the valence orbital diagram that represents the ground state of Sr2⁺. asked Jul 31, 2019 in Chemistry by ... Choose the valence orbital diagram that represents the ground state of Br-. asked Jul 31, 2019 in Chemistry by johnb. general-chemistry; Choose the valence orbital diagram that represents the ground state of Ni. asked Jul 31 ...
Page 2 A)Li B)Si C)Al D)Cl 13.The diagram below represents the orbital notation of an atom's valence shell in the ground state. The diagram could represent the valence shell of
Choose The Valence Orbital Diagram That Represents The Ground State Of Zn. The partial orbital diagram below where n could be any valid quantum number. 11 years aieee chapterwise by mtg hope it helps by shreesha rao 2 in types school work the d block elements lardbucket alloys and pounds of the d block elements are important ponents of the ...
Study Chem 170 Chapter 8 flashcards from Brendan Jackson's class online, or in Brainscape's iPhone or Android app. Learn faster with spaced repetition.
Example: 1s 2 For writing ground state electron configurations, a few main steps should be followed. Find the amount of electrons in the atom. Example: Na: 11 e - Na +: 10 e -. Fill orbitals following the model until all electrons have been accounted for. Example: Na: 11 e - 1s 2 2s 2 2p 6 3s 1 or Na +: 1s 2 2s 2 2p 6.








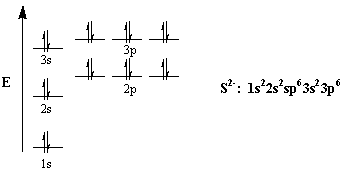












0 Response to "40 choose the valence orbital diagram that represents the ground state of se2-."
Post a Comment