40 electrolysis of water diagram
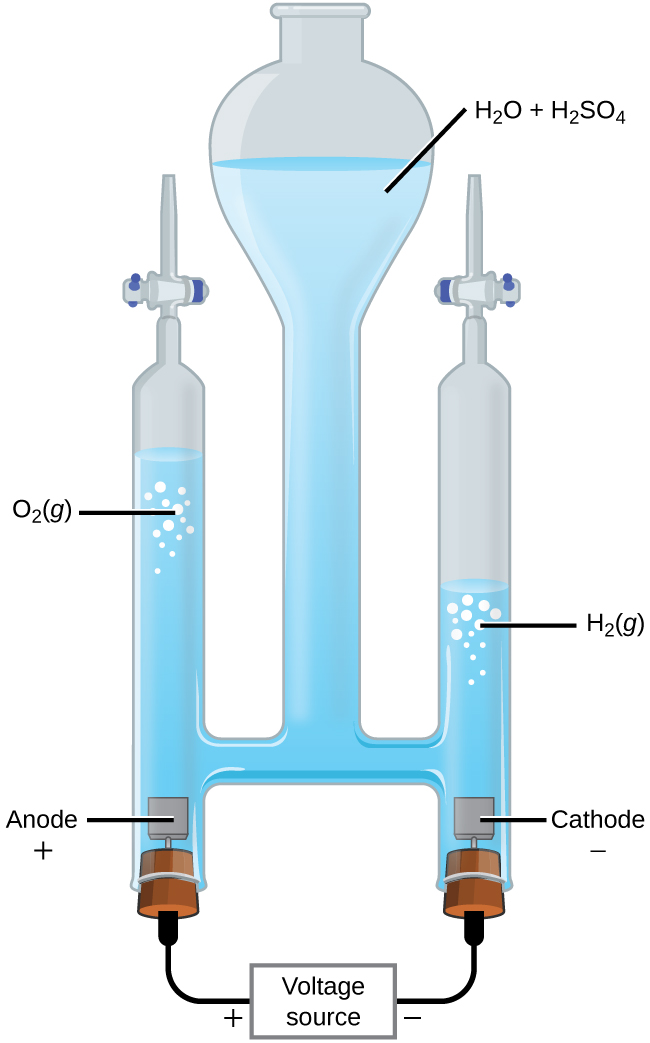
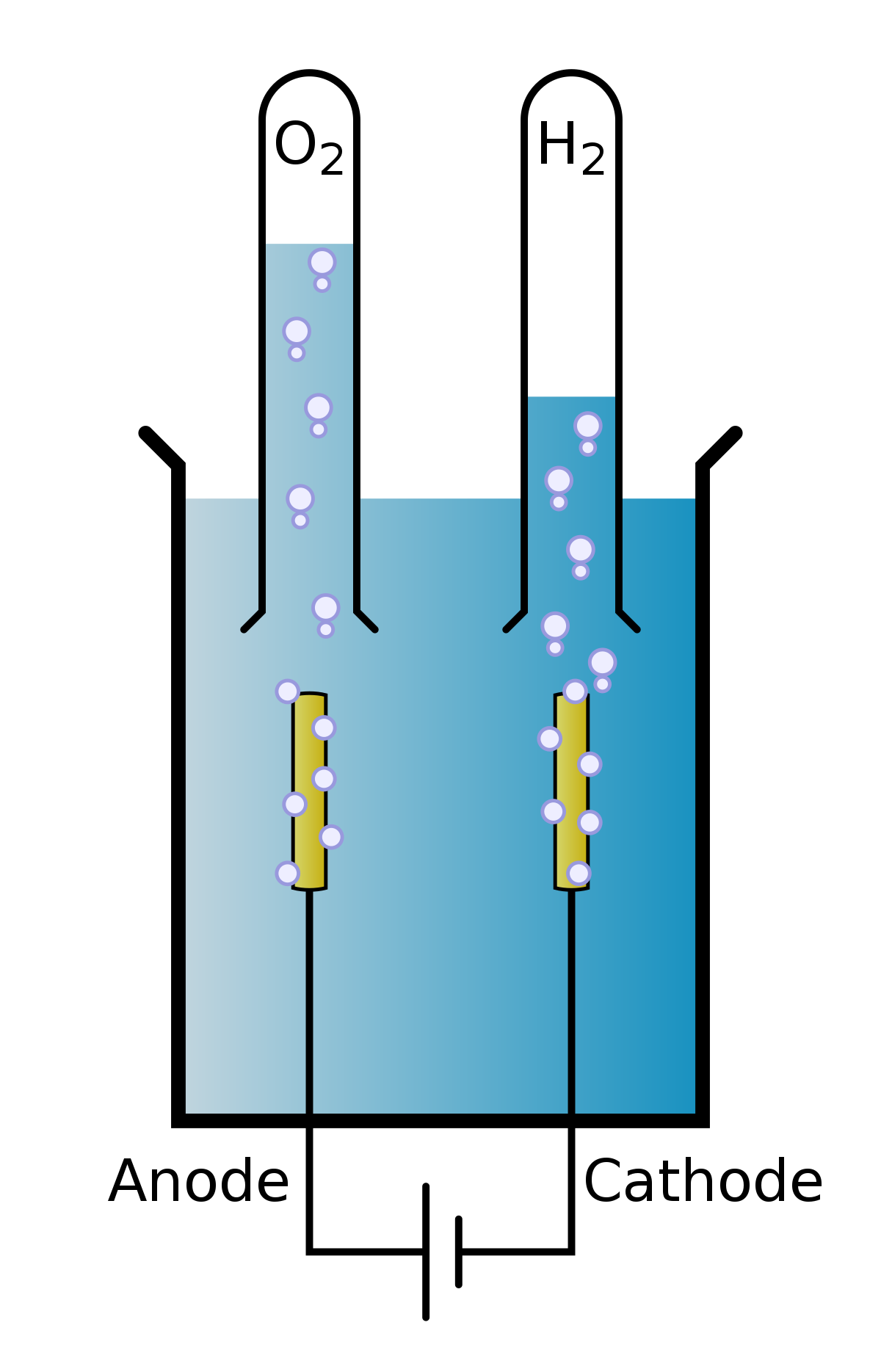
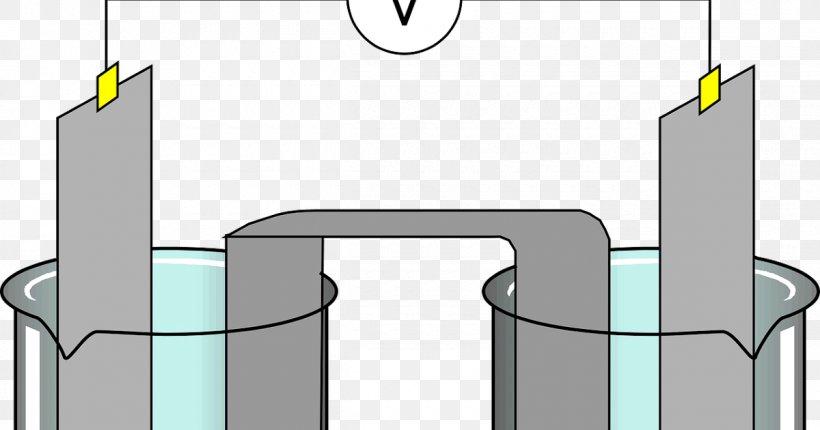
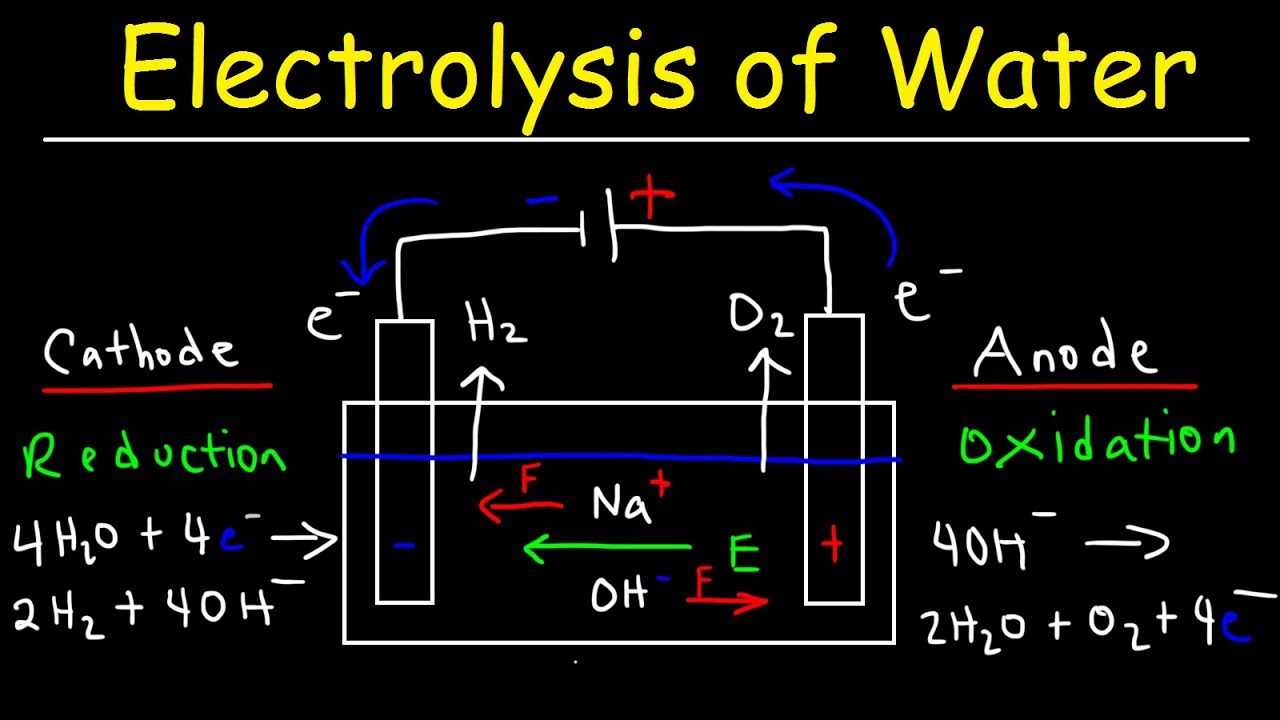
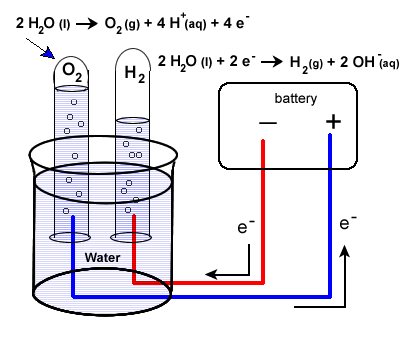
Illustration about Labeled diagram to show the electrolysis of acidified water forming hydrogen and oxygen gases. Illustration of diagram, acidified, electrode - 61461102 During electrolysis of pure water, at the negatively charged cathode, a reduction reaction takes place, with electrons from the cathode being given to hydrogen cations to form hydrogen gas (the half-reaction balanced with acid): Cathode (reduction): 2 H + + 2 e − → H 2 Anode (oxidation): 4 O H − → O 2 + 2 H 2 O + 4 e −
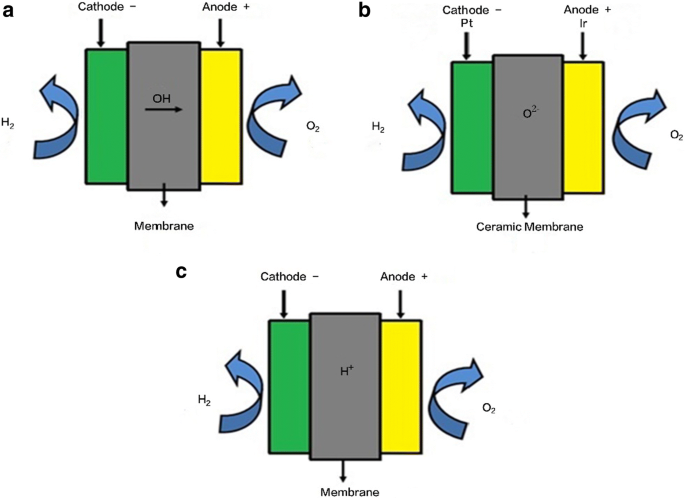
Water electrolysis technology is relatively mature and the equipment is complete and systematic (Fig. 7.2).The production of hydrogen by electrolyzing water accounts for about 4% of total hydrogen production (Mazloomi and Gomes, 2012).There are three main types of electrolyzers in water electrolysis hydrogen production process technology: alkaline electrolytic cells, polymer electrolytic cells ...
Electrolysis of water diagram
Electrolysis of Water Experiment. Energy is stored in the bonds of molecules. When these bonds split apart, the energy released can be used to do work. Breaking apart liquid water molecules into hydrogen and oxygen gas creates an enormous amount of energy, which can be turned into useful electricity to power our homes and cars. From the diagram and equations, we can see that the volume of hydrogen produced is double the volume of oxygen. This confirms that there are twice as many hydrogen atoms as oxygen atoms in water ... 2. Theory of water electrolysis The electrolysis of water is considered a well-known principle to produce oxygen and hydrogen gas. In Fig.1 a schematic of an electrochemical cell is presented. The core of an electrolysis unit is an electrochemical cell, which is filled with pure water and has two electrodes connected with an external power supply.
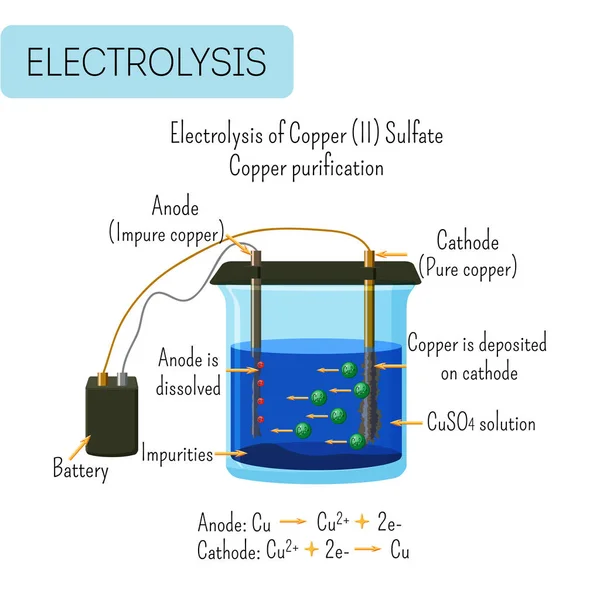
Electrolysis of water diagram. Hello Everyone.Diagram of Electrolysis of Water || How To Draw Labelled Diagram of Electrolysis of WaterDiagram of Electrolysis of Water, How To Draw Labelle... Q.4. Which electrodes are used in the electrolysis of water? Ans: Platinum electrodes are used in the electrolysis of water. Q.5. What is the conclusion of the electrolysis of water? Ans: The conclusion of the electrolysis of water is that hydrogen gas is produced at the cathode, whereas oxygen gas is produced at the anode. Q.6. In molten sodium chloride, the ions are free to migrate to the electrodes of an electrolytic cell. A simplified diagram of the cell commercially used to produce sodium metal and chlorine gas is shown in Figure 1. Sodium is a strong reducing agent and chlorine is used to purify water, and is used in antiseptics and in paper production. Electrolysis of Water . Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. By adding electricity to water and providing a path for the different particles to follow, water can be separated into hydrogen and oxygen. In this experiment you will be taking a sample of salt water and adding a ...
Electrolysis of dilute sulfuric acid The products of electrolysing water acidified with sulfuric acid are hydrogen gas and oxygen gas Two experimental setups are described, the Hofmann voltameter demonstration (left diagram) and a simple cell (right diagram) for use in schools and colleges for pupils to use. Dilute sulfuric Electrolysis of Water: With the ever-increasing need for fossil fuels, scientists are always looking for new ways to generate energy. Thanks to hydrogen, which does not pollute the air when burned. Finding cost-effective ways to create hydrogen has been a challenge. ADVERTISEMENTS: Electrolysis: Definition and Uses (explained with diagram)! While passing electricity through electrolytes, you might have noticed some changes in them. You might also have noticed bubbles of gas at the electrodes as well as changes in the metal electrodes. ADVERTISEMENTS: All these indicate that when an electric current is passed through an electrolyte, a […] Alkaline water electrolysis uses fresh water with low salt content, and hence additional treatment and desalination sys-tems add to the cost of hydrogen produced. Fig. (2) shows two established technologies of electrolysis, alkaline water electrolysis and brine electrolysis. In the former, hydrogen is the main product, while in the
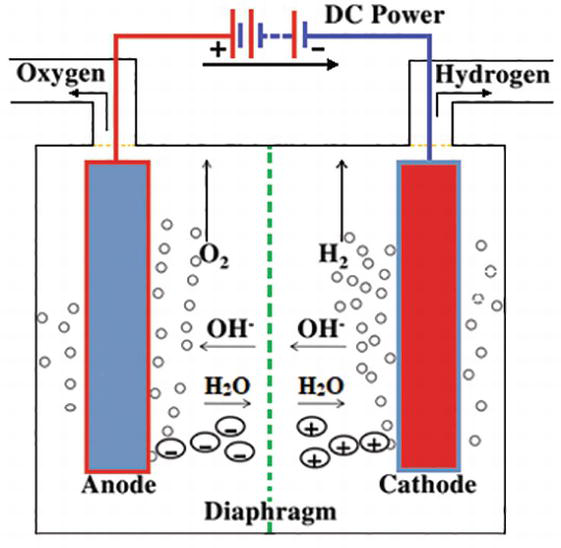
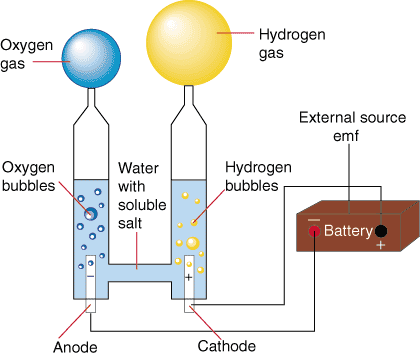
2 We will analyze a 1 GW (200,000 Nm3/hr / 500 ton H2 per day) hydrogen electrolysis plant capex. 2019 YY Project Objective Total Project Cost Stack + BOP Factory Cost = X OEM Markup Additional Project + Cost Electrolysis Plant CAPEX Electrolysis of water can be achieved in a simple hands-on project, where electricity from a battery is passed through a cup of water (in practice a saltwater solution or other electrolyte will need to be used ... On their diagram, indicate which ions are located near the cathode and the anode. Electrolysis of Water. The electrolysis of water produces hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as H 2 SO 4 has been added. The electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. The first commercialized water electrolysis system was based on the principles of alkaline water electrolysis, and alkaline-based systems remain the most ubiquitously utilized water electrolysis systems [17]. A schematic of an alkaline water electrolyzer is given in Fig. 16.3.
(Hint: Water’s chemical name is H 2 O because it has two hydrogen atoms to every one oxygen atom.) Further experimenting: Try adding an electrolyte to the water in the beaker. Water doesn’t conduct electricity that well by itself, but any electrolysis of water experiment could be accelerated by adding table salt to the water.
Start studying Electrolysis of Water. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Water Electrolysis Process, Scientific Chemistry Diagram, Vector Illustration Educational Poster with power source, water, gases and chemical elements scheme. Labeled diagram to show the electrolysis of acidified water forming hydrogen and oxygen gases.
Figure 2. ∆G-T diagram of water splitting. Because of the endothermic reaction the energy that is equal to ΔH should be provided to split water into hydrogen and oxygen; ΔG as a useful work and TSΔ as a thermal GT diagram for the water splitting reaction is shown in Figure 2. The water decomposition reaction has a large positive free ...
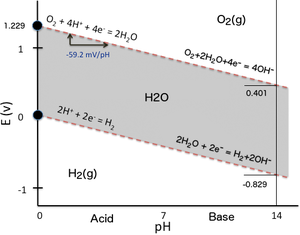
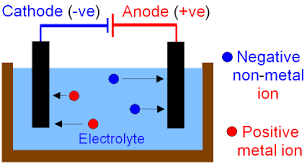
Pourbaix diagram gives the equilibrium regions of water, hydrogen and oxygen at various electrode potentials. Water Electrolysis in the Presence of Salts Salts are 100%dissociate into cations and anions in water and hence increase the ionic concentration for increasing conductivity.
Separating the word "electrolysis" into its component parts summarizes its meaning—using electricity (electro-) to break apart (-lysis) something. In this demonstration, the electricity supplied by a 9-volt battery is used to break apart water molecules, overall producing hydrogen and oxygen gases.
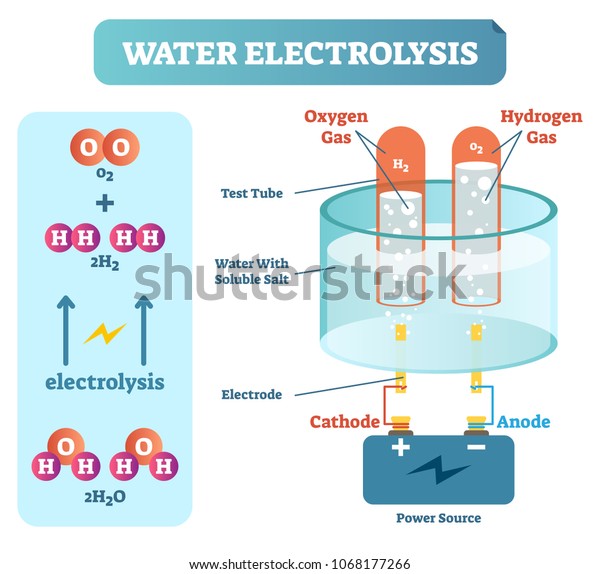
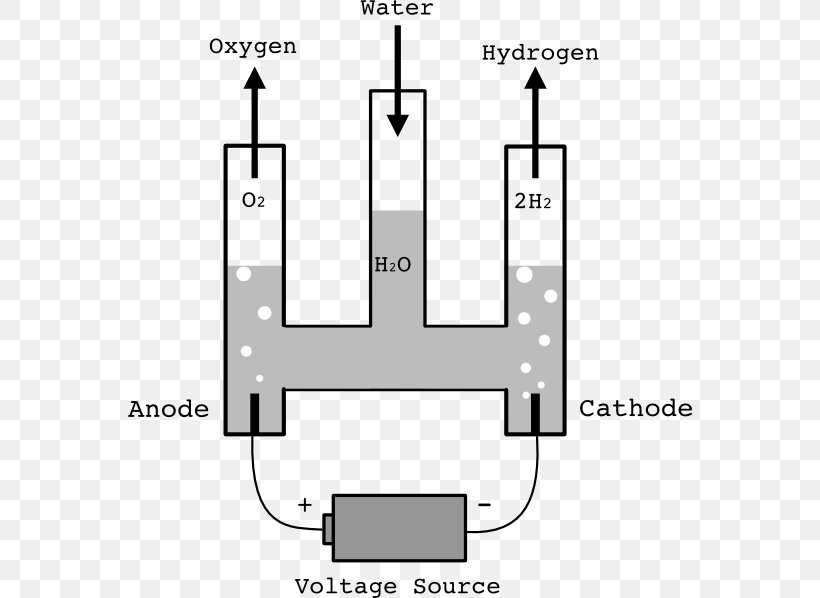
Electrolysis of water is the decomposition of water (H 2 O) into oxygen (O 2 ) and hydrogen gas (H 2 ) due to an electric current being passed through the water.An electrical power source is connected to two electrodes, or two plates (typically made from some inert metal such as platinum, stainless steel or iridium) which are placed in the water.
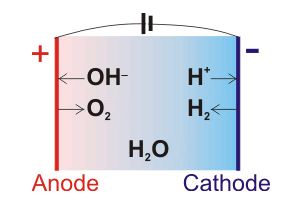
Electrolysis of Water (A teacher demonstration experiment) Lab # 1 Background Electrolysis of water is the process by which water is decomposed into oxygen and hydrogen gas, when electric current is passed through it. Water molecule is decomposed in to H+ and OH- ions, when electric current is passed through it.
A diagram of the electrolysis of water. Electrolysis of water is the decomposition of water into oxygen and hydrogen gas. This technique can be used to produce breathable oxygen or hydrogen gas as a fuel source, referred to as hydrogen fuel. However, that method is more expensive than the industrial way to produce hydrogen fuel from natural gas.
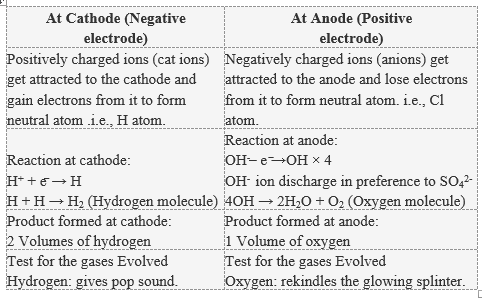
Dissociation reaction: 2H 2 O 2H 2 + O 2. Acidified water Hydrogen gas Oxygen gas. Discharge of Ions at Electrodes. At Cathode (Negative. electrode) At Anode (Positive. electrode) Positively charged ions (cations) get attracted to the cathode and gain electrons from it to form neutral atom. i.e, H atom.
Draw a neat labelled diagram of electrolysis of water. - Get the answer to this question and access more number of related questions that are tailored for students.
Electrolysis refers to the use of electricity to drive a chemical reaction that would not normally occur on its own. In this lab, you will build an electrolytic cell – an apparatus for carrying out electrolysis and use it to produce various gases and other chemical compounds. A diagram of a basic electrolytic cell is shown here.
Electrolysis is a promising option for carbon-free hydrogen production from renewable and nuclear resources. Electrolysis is the process of using electricity to split water into hydrogen and oxygen. This reaction takes place in a unit called an electrolyzer.
This chemistry video tutorial provides a basic introduction into the electrolysis of water which splits H2O into H2 (hydrogen has) and O2 (oxygen gas). Oxyg...
Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas by a process called electrolysis.Hydrogen gas released in this way can be used as hydrogen fuel, or remixed with the oxygen to create oxyhydrogen gas, which is used in welding and other applications.. Sometimes called water splitting, electrolysis requires a minimum potential difference of ...
2. Theory of water electrolysis The electrolysis of water is considered a well-known principle to produce oxygen and hydrogen gas. In Fig.1 a schematic of an electrochemical cell is presented. The core of an electrolysis unit is an electrochemical cell, which is filled with pure water and has two electrodes connected with an external power supply.
From the diagram and equations, we can see that the volume of hydrogen produced is double the volume of oxygen. This confirms that there are twice as many hydrogen atoms as oxygen atoms in water ...
Electrolysis of Water Experiment. Energy is stored in the bonds of molecules. When these bonds split apart, the energy released can be used to do work. Breaking apart liquid water molecules into hydrogen and oxygen gas creates an enormous amount of energy, which can be turned into useful electricity to power our homes and cars.




















0 Response to "40 electrolysis of water diagram"
Post a Comment