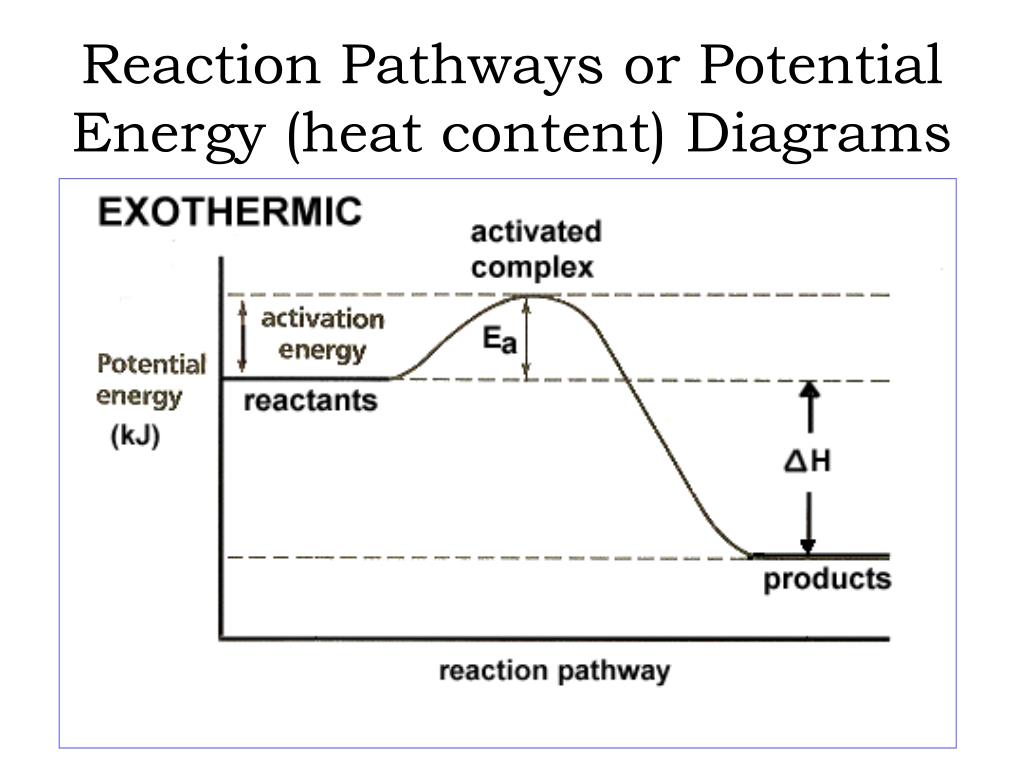
42 potential energy diagram exothermic
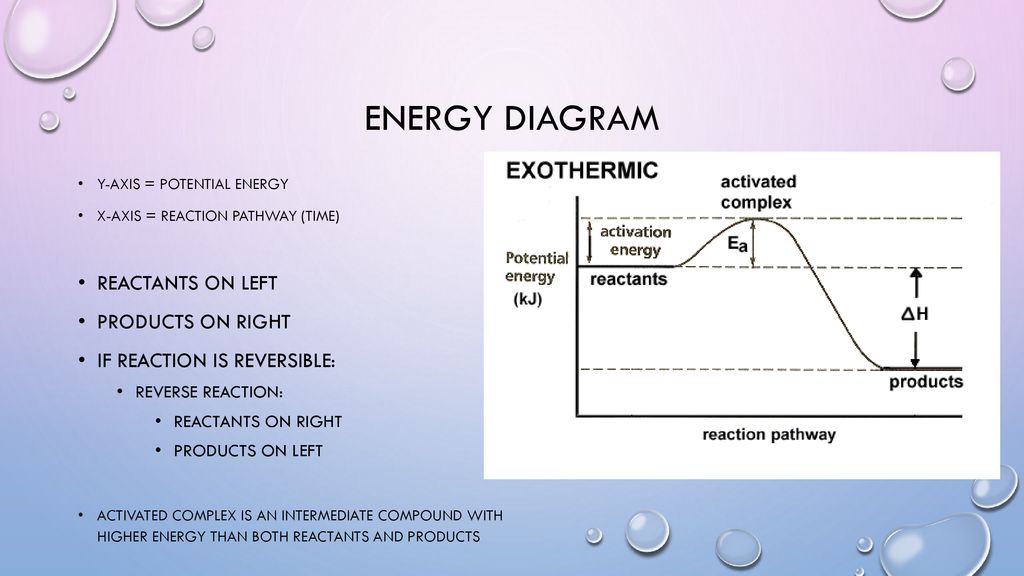
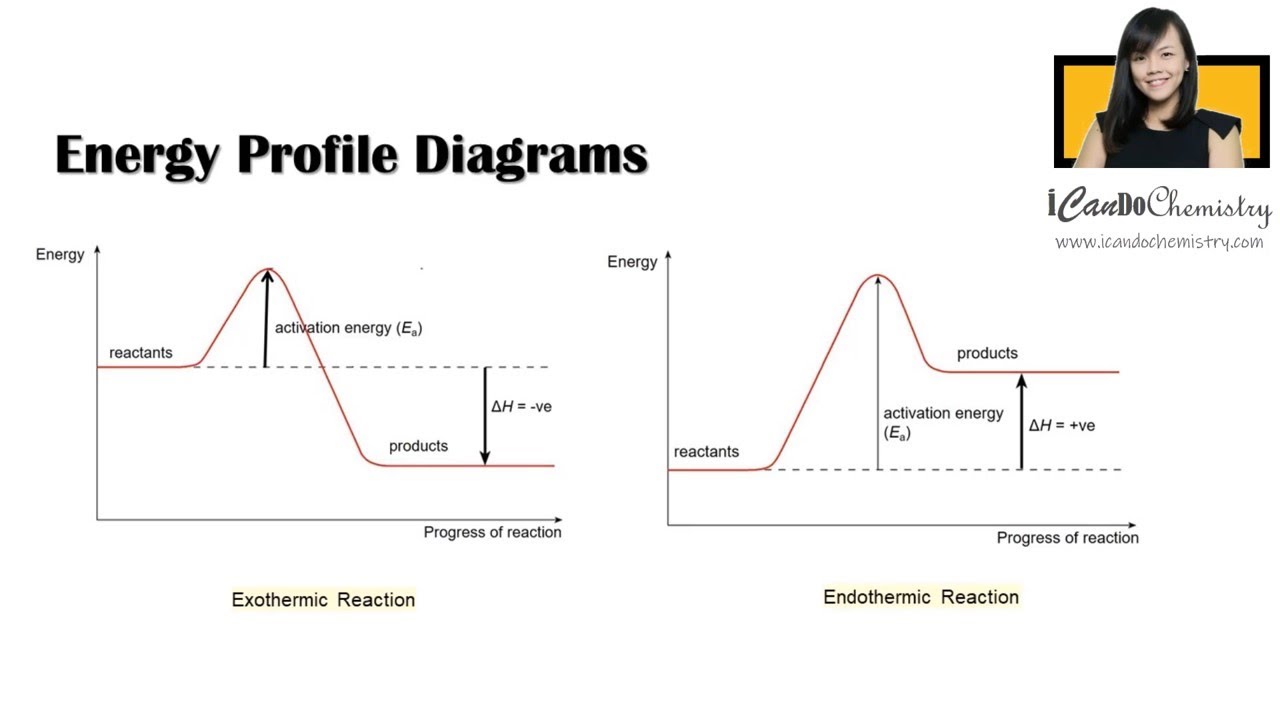
is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the potential energy diagrams. The total potential energy ... ENERGY DIAGRAM. • A = POTENTIAL ENERGY OF REACTANTS. • B = ACTIVATION ENERGY. • ENERGY REQUIRED TO START THE REACTION. • C = ACTIVATION ENERGY (REVERSE.11 pages
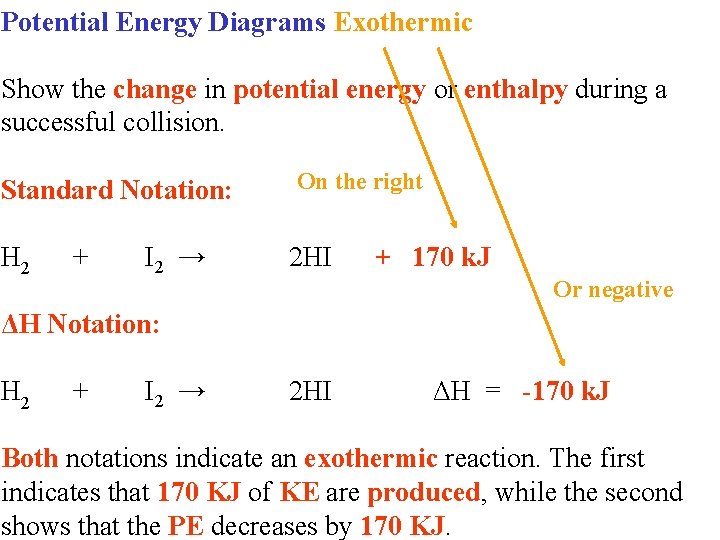
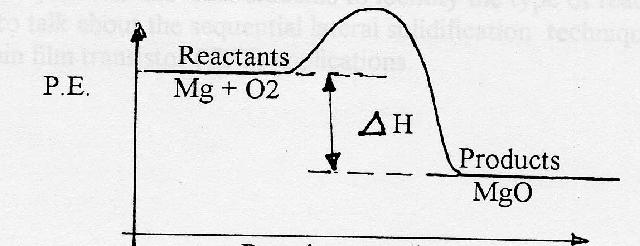
Potential energy diagram worksheet answers 1. δh pep per. The overall difference in potential energy between the products and the reactants. Is the reaction in 6 exothermic or endothermic. Every chemical reaction will either absorb or release energy. For a given reaction the.

Potential energy diagram exothermic
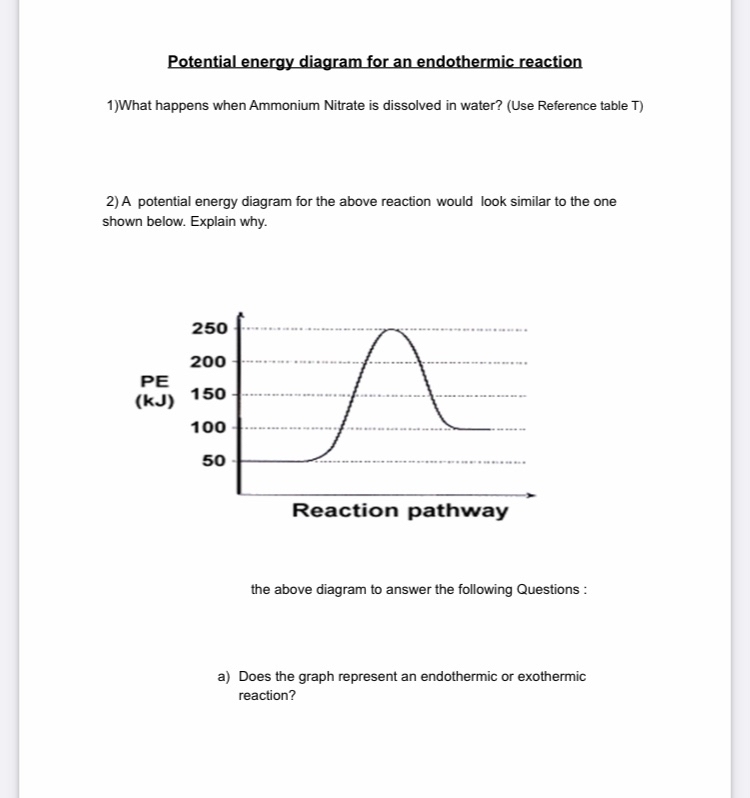
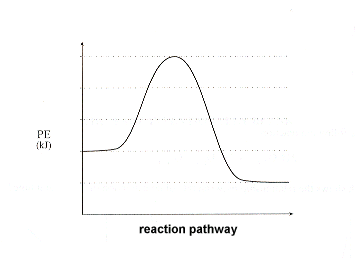
The potential energy diagrams for the exothermic reaction between substances X and Y is shown below. Worksheet 1-2 Potential Energy Diagrams Page 2. Potential Energy Diagrams Aimver the questions by nferrixig to the diagmm of Aep enezsF of o reaction. Energy the solar system even the periodic table are all forms of physical science. A student made a sketch of a potential energy diagram to represent an exothermic reaction. A curve line graph is shown. The y axis of the graph has the title Potential Energy and kJ written in parenthesis. The x axis of the graph has the title Reaction Pathway. The curve begins at a lower level and ends at a slightly higher level. For the above Engothermic Potential Energy Diagram: 1. Forward Activation Energy is 250kJ-50kJ = 200kJ. 2. Potential Energy of the Products = 100kJ. 3. Potential Energy of Reactants = 50kJ. 4. Forward Heat of Reaction = 100kJ - 50kJ = 50kJ (Notice the result is positive) Know the different parts of both the Exothermic and Endothermic Potential ...
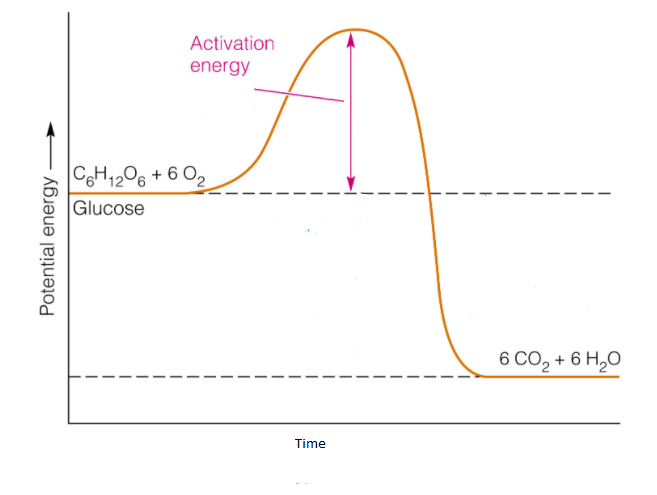
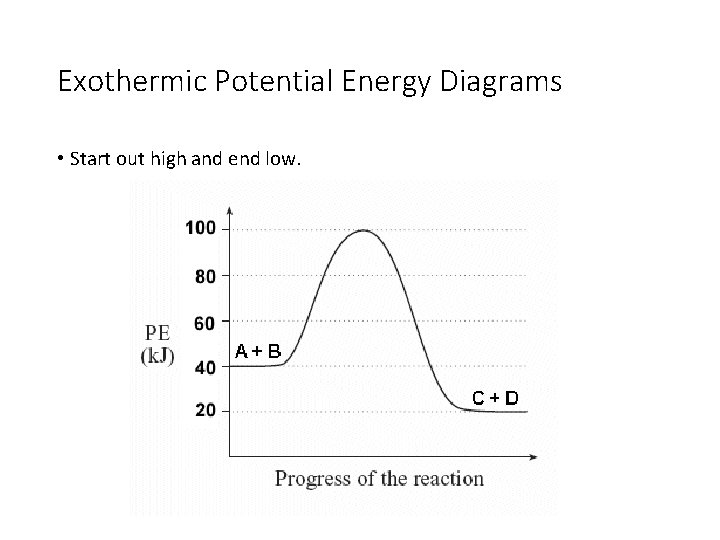
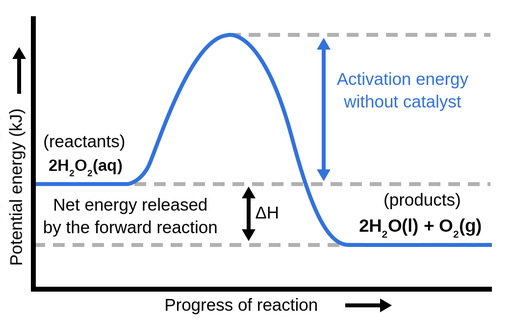
Potential energy diagram exothermic. Jul 9, 2019 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ... Potential Energy Diagram Practice Endothermic And Exothermic Reactions Potential Energy Energy Activities Exothermic Reaction . In the scope of its subject chemistry occupies an intermediate position. Potential energy diagram worksheet chemistry. The potential of a cell measured in volts is the energy needed to move a charged particle in an ... 1. Answer the following questions based on the potential energy diagram shown here: Does the graph represent an endothermic or exothermic reaction? Label the position of the reactants, products, and activated complex. Determine the heat of reaction, ΔH, (enthalpy change) for this reaction. Determine the activation energy, E a for this reaction. In an exothermic reaction, the products have less potential energy than the reactants had. This is why the potential energy diagram for an exothermic reaction starts at a higher energy value and ends at a lower energy value. In an endothermic reaction, the products end up with more stored potential energy than the reactants.
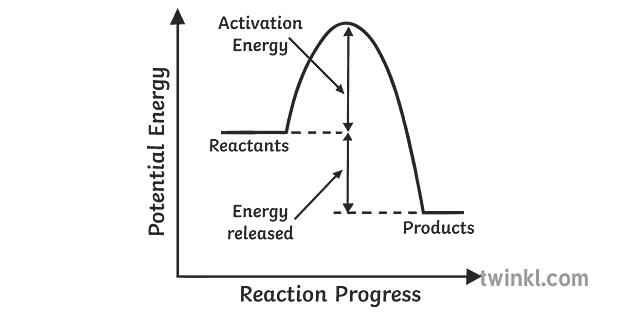
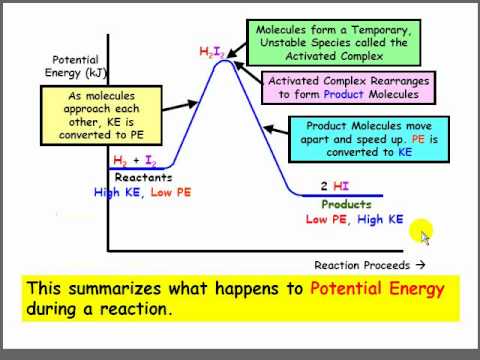
A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change \(\left( \Delta H \right)\) is positive for an endothermic reaction and negative for ... Potential energy diagrams are useful in observing the potential energy changes during a reaction process. Usually, potential energy diagrams were plotted using reaction pathway/reaction progress in the x-axis and potential energy in the y-axis. For exothermic reactions, the total energy of the products will be leass than the reactants. Since ... A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. Potential energy diagrams for endothermic and exothermic reactions are described. Diagrams of activation energy and reaction progress are given. 1 Potential Energy Diagrams Worksheet Potential Energy Diagrams Worksheet WARM UP: Activation energy is the energy a reaction needs to get started. At the bottom of the page are two energy diagrams — one for an exothermic reaction and one for an endothermic reaction. Follow the directions below to label the energy diagrams. 1.
The energy required to form the intermediate, called activated complex is known as activation energy . The diagram is obtained by plotting potential energy vs. reaction coordinate. Reaction coordinate represents the profile of energy change when reactants change into products. <br> Some energy is released when the complex decomposes to form ... Energy profiles or energy diagrams for endothermic and exothermic reactions with or without a catalyst tutorial with worked examples for Chemistry students. An exothermic reaction is one in which heat energy is given out. The difference between the potential energies of products and reactants gives the heat of reaction. An energy diagram can be defined as a diagram showing the relative potential energies of reactants, transition states, and products as a reaction progresses ... Draw the potential energy diagram for an exothermic reaction. Explain the terms: <br> activation energy of backward reaction . Updated On: 21-7-2021. To keep watching this video solution for FREE, Download our App. Join the 2 Crores+ Student community now! Watch Video in App Continue on Whatsapp.
Since the potential energy of the products is higher than the potential energy of the reactants, the reaction is endothermic and the enthalpy (E(C)-E(A)) has a positive sign.The activation energy (E(B)-E(A)) is positive as well.. We have a diagram showing the potential energy changes for a reaction pathway.. Point A represents the potential energy of the reactants.
Strong bonds have lower potential energy than weak bonds. An energy diagram represents this change. This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It shows the energy in the reactants and products and the difference in energy between them. More rigorous Gibbs free energy spontaneity ...
A certain reaction happens in two steps. In the first step, A forms B and is slightly endothermic with a high activation energy barrier. In the second step, B forms C + D, which is highly exothermic with a very low activation energy barrier. Sketch the potential energy diagram for this two-step reaction.
For the above Engothermic Potential Energy Diagram: 1. Forward Activation Energy is 250kJ-50kJ = 200kJ. 2. Potential Energy of the Products = 100kJ. 3. Potential Energy of Reactants = 50kJ. 4. Forward Heat of Reaction = 100kJ - 50kJ = 50kJ (Notice the result is positive) Know the different parts of both the Exothermic and Endothermic Potential ...
A student made a sketch of a potential energy diagram to represent an exothermic reaction. A curve line graph is shown. The y axis of the graph has the title Potential Energy and kJ written in parenthesis. The x axis of the graph has the title Reaction Pathway. The curve begins at a lower level and ends at a slightly higher level.
The potential energy diagrams for the exothermic reaction between substances X and Y is shown below. Worksheet 1-2 Potential Energy Diagrams Page 2. Potential Energy Diagrams Aimver the questions by nferrixig to the diagmm of Aep enezsF of o reaction. Energy the solar system even the periodic table are all forms of physical science.





















0 Response to "42 potential energy diagram exothermic"
Post a Comment