44 no2- molecular orbital diagram
This problem has been solved! See the answer. See the answer See the answer done loading. NO2+ molecular orbital diagram. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (1 rating) According to Electronic Structure of NO2 Studied by Photoelectron and Vacuum-uv Spectroscopy and Gaussian Orbital Calculations J. Chem. Phys. 53, 705 (1970) : The highest molecular orbital 4 a 1 is occupied by the 1 unpaired electron. Experimentally, Oxides and Oxyions of the Non-metals.
The molecular orbital diagram for ClO - is given below: The basis orbitals for Cl are 3s and 3p and for O are 2s and 2p. Z* for O 2s and 2p orbitals are similar so the AOs start at nearly the same energy. For the Cl 3s and 3p orbitals the two Z* values are quite different so the initial energies are more separated.
No2- molecular orbital diagram
You've seen the molecular orbital (MO) diagram of "CO"_2: "CO"_2 and "NO"_2^+ are isoelectronic and thus have the same electron configuration. Thus, simply add one or two electrons into the 2b_(3u) and 2b_(2u) to get "NO"_2 and "NO"_2^-, respectively. Nitrogen atom has 2p atomic orbitals lower by "2.52 eV", and 2s atomic orbitals lower by "6.13 eV" than with carbon atom. In both molecules the pi symmetry molecular orbitals are the same. The 2p x orbitals on each atom combine to make a pi bonding and a pi antibonding molecular orbital in the xz plane. Perpendicular to these in the yz plane, the 2p y orbitals on each atom combine to make a pi bonding and a pi antibonding molecular orbital. Here is the full molecular orbital diagram for N 2. Nitrogen dioxide is a chemical compound with the formula NO 2.It is one of several nitrogen oxides. NO 2 is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the production of fertilizers.At higher temperatures it is a reddish-brown gas. It can be fatal if inhaled in large quantity.
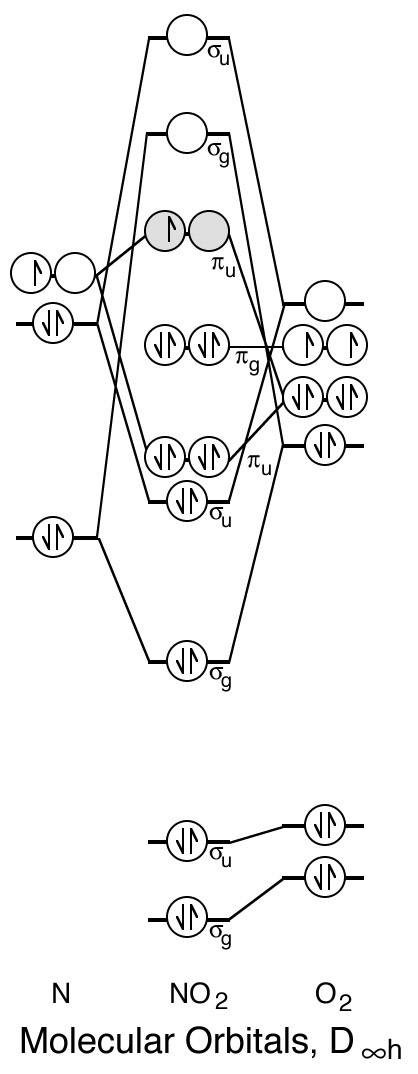
No2- molecular orbital diagram. 21.11.2021 · N2O4 Molecular Orbital (MO) Diagram. A molecular orbital (MO) diagram explains the chemical bonding in molecules by energy level diagrams. They were proposed by Robert S. Mulliken and Friedrich Hund in 1928. As we know N2O4 molecule is a dimer of the NO2 molecule, hence we’ll discuss molecular orbital diagram of NO2 molecule first. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Printable O2 molecular orbital diagrams are available for you to guide your study in the molecular orbital lesson.This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. 9. An element has a body centered cubic (bcc) structure with a cell edge of 288 pm. The atomic radiusis: NEET 2020 The Solid State. 10. Find out the solubility of N i ( O H) 2 in 0.1 M NaOH. Given that the ionic product of N i ( O H) 2 is 2 × 10 − 15. NEET 2020 Equilibrium.
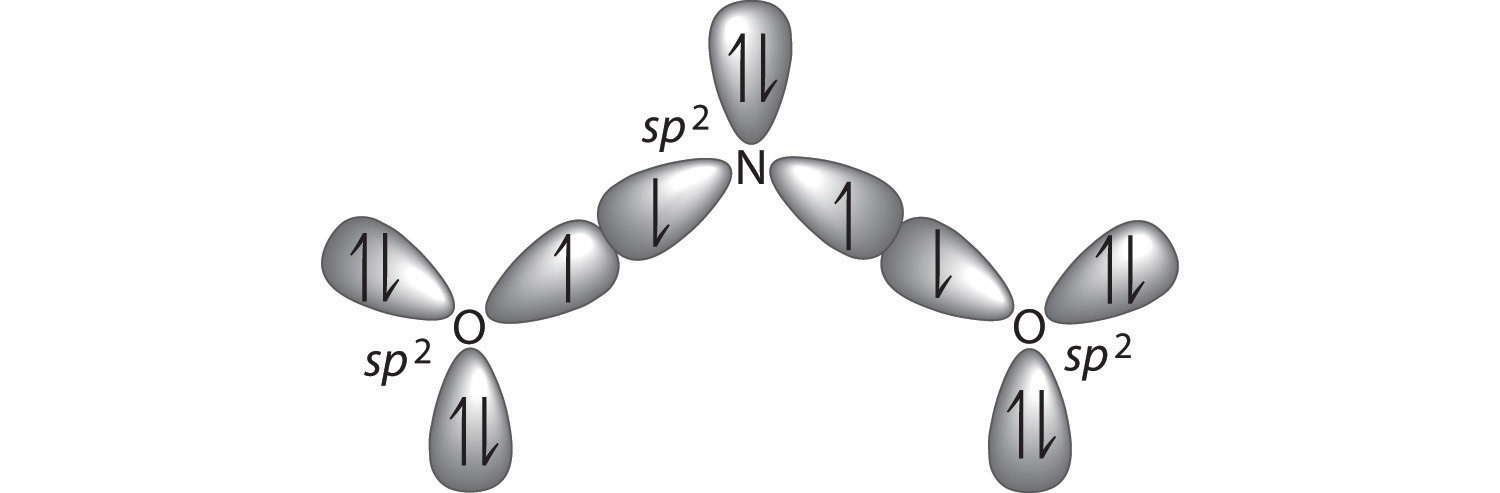
Asked for: bonding description using hybrid atomic orbitals and molecular orbitals. Strategy: Calculate the number of valence electrons in NO 2 −. From the structure, predict the type of atomic orbital hybridization in the ion. Predict the number and type of molecular orbitals that form during bonding. Hybridization of NO2 (Nitrogen Dioxide) NO 2 involves an sp 2 type of hybridization. The most simple way to determine the hybridization of NO 2 is by drawing the Lewis structure and counting the number of bonds and lone electron pairs around the nitrogen atom. You will find that in nitrogen dioxide there are 2 sigma bonds and 1 lone electron pair. The Nitrogen atom in the Lewis structure for NO 2 is the least electronegative atom and passes at the centre of the structure. (Image to be added soon) Molecular Geometry and Bond Angles of NO 2. Since the Nitrogen Dioxide (NO 2) has an extra electron in a nitrogen atom's orbital, it will result in a higher degree of repulsions. However, if ... 21.11.2021 · 77) Based on molecular orbital theory, the only molecule in the list below that has unpaired electrons is _____. 9° and the HCH bond angle is 116. Since there are six Fluorine (F) atoms it will be necessary. trigonal-planar Explanation: geometry of SeF6 = ? The Lewis structure for SeF6 is. Lewis Formulas. View this answer. H 2 O bent, polar m. The Lewis diagram is as follows: N = 5 e-O = 6e ...
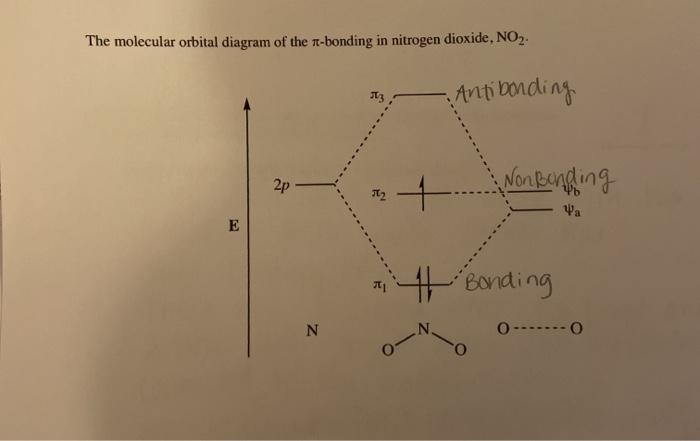
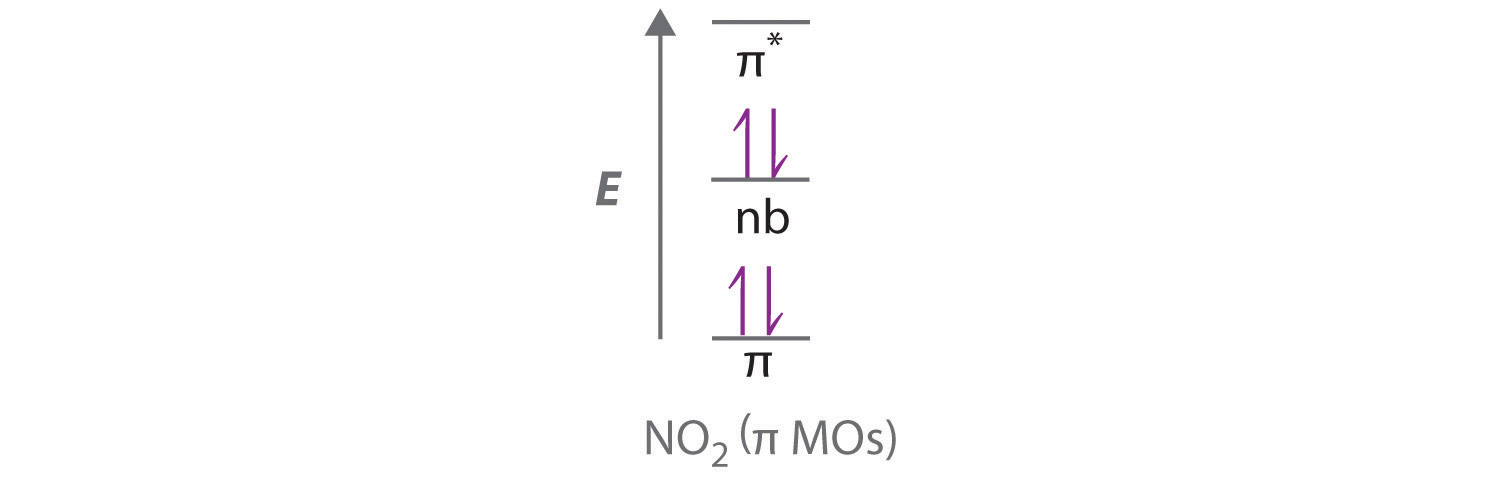
The molecular orbital energy level diagram of oxygen molecule is given as follows : Bond order 2 N b − N a = 2 8 − 4 = 2 Thus, oxygen molecule has two bonds. i.e., one is bond and one p bond. Answer to: The reaction A=B + C is second order in A. When [A}o = 0.100 M, the reaction is 20.0% complete in 37.7 minutes. Calculate the value of... The correct orbital diagram for the valence electrons of silicon is. the pic that has three lines filled up. oneup down other two are just upup . Which of the following substances contains both ionic and covalent bonds? NaOH. The photoelectron spectra above show the energy required to remove a 1s electron from a nitrogen atom and from an oxygen atom. Which of the following statements best ... 30.11.2021 · Also, we have the concept of HOMO ( Highest Occupied Molecular Orbital) and LUMO ( Lowest Unoccupied Molecular Orbital). MO Diagram for NO2. Let us look at the electronic configuration of both N & O. N: 1s2 2s2 2p3 O: 1s2 2s2 2p4 ( we have two O atoms in NO2 ) The six electrons present in the 1s orbital do not take part in bonding, therefore will play the role of non-bonding orbitals. The …
Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ...
Figure 10.5. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Part (a) in Figure 10.5. 3 shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The result is a slight change in the relative energies of the molecular orbitals, to give the diagram shown in the figure below.
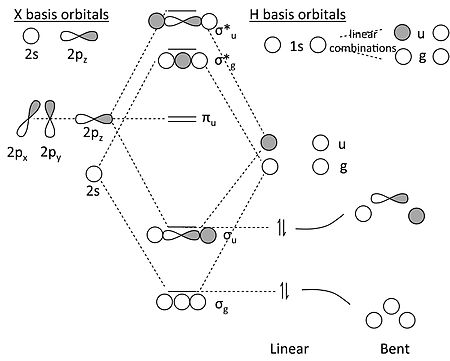
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
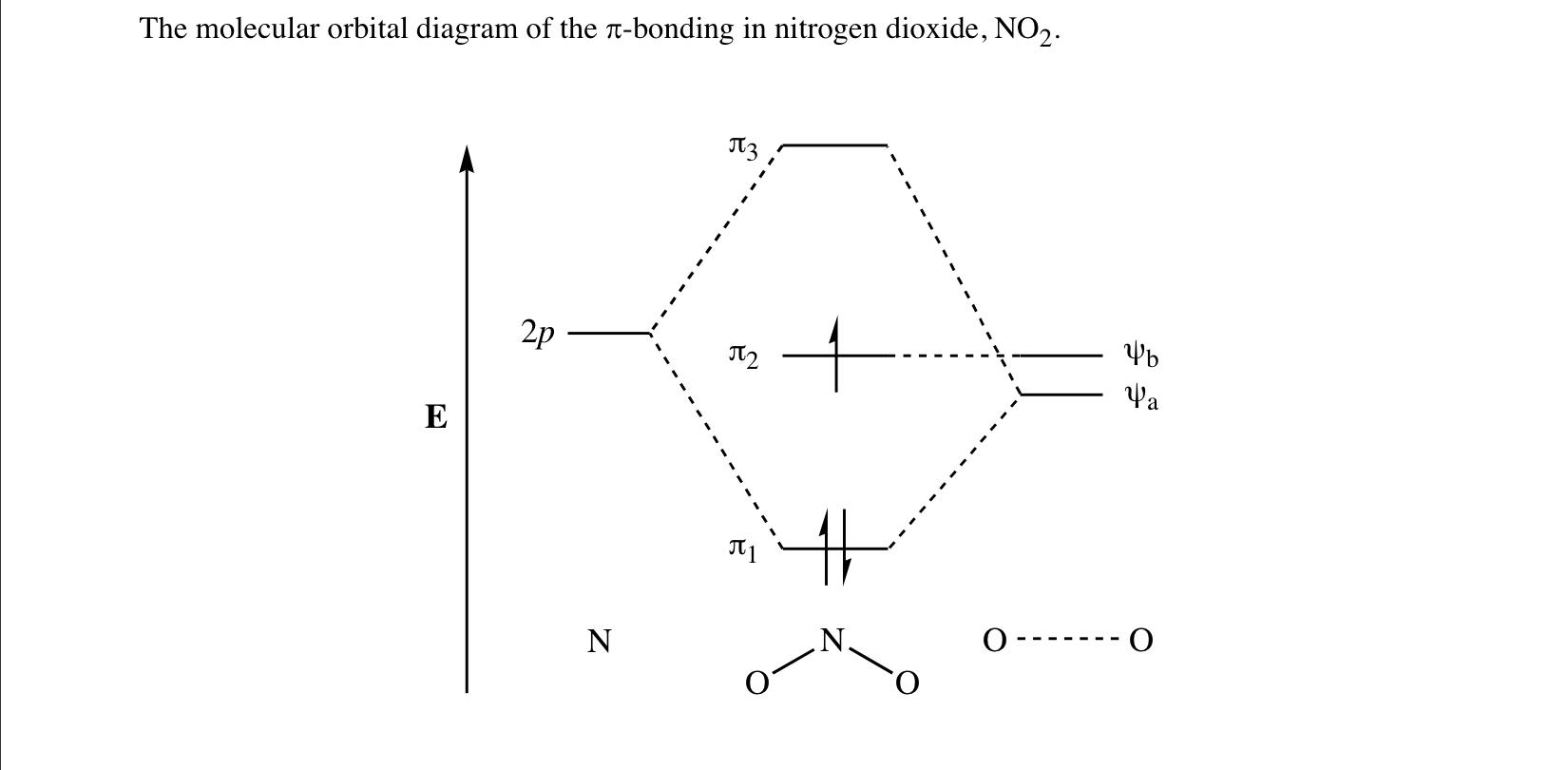
Answer (1 of 2): This image shows the molecular orbitals of nitric oxide and the types of bonds present.
Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
Answer: I think you can safely assume to start off with the molecular orbital diagram of the Nitrite anion (NO₂¯) and then remove an electron from it: What will be the molecular orbital diagram for nitrite ion? The outcome, i.e. the molecular orbital diagram for Nitrogen dioxide NO₂, should loo...
Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*...
Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown:(i) Electronic configuration:(ii) Bond order: Here Nb = 8; Na = 4The two oxygen atoms in a molecule of oxygen are united through two covalent bonds ...
The bonding orbitals are slightly more concentrated on o. Pdf Lewis Acidity Of No And No2 As Measured By Their Affinity To These are uvvisible infra red ir and nuclear magnetic resonance nmr spectroscopies. No molecular orbital diagram. Now draw two more mo diagrams for no and no your no diagram will have one less valence electron and your no ...
NO2-1, CO3-2, CH3COO-1 9-5 Electronegativity and Polar Covalent Bonding Correct the following statement: “The bonds in solid PbCl 2 are ionic; the bond in a HCl molecule is covalent. How many covalent bonds will a nitrogen atom usually form? a. framework cs6 mac acrobat crack zip 2 girls wearing diaper inside pyjama, DG5 @iMGSRC. Thus OsO 4 is osmium tetroxide rather than osmium …
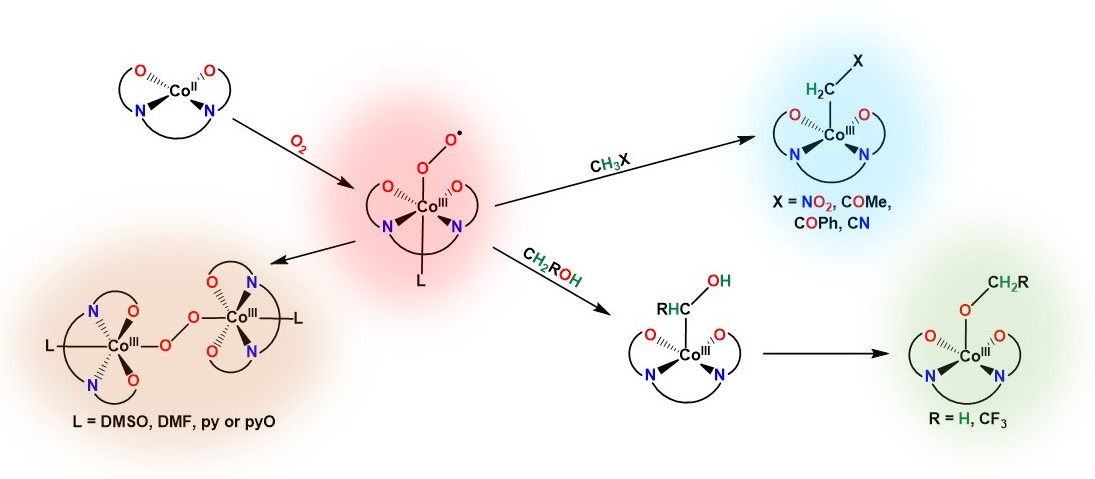
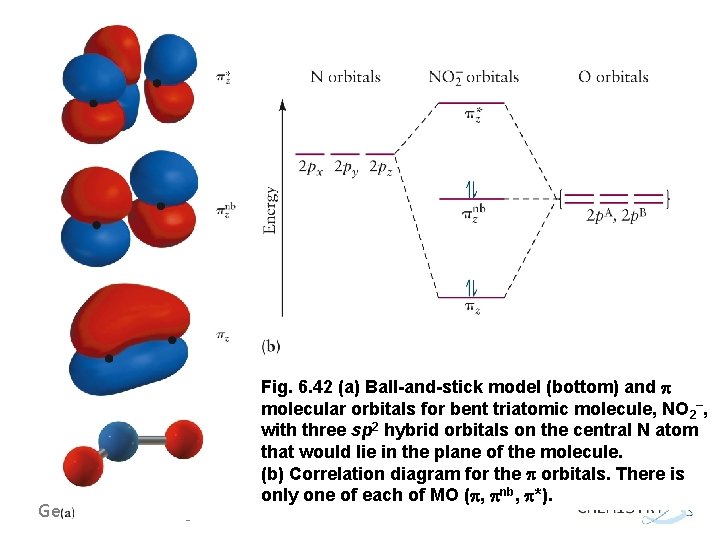
Molecular orbitals in NO 2 Will the molecule be linear or bent? Click on a color picture to watch the geometry change from linear to bent. 2흅u: 2b 1 6a 1 : 1흅g: 1a 2 4b 2 : 1흅u: 1b 1 5a 1: Movies on this page were created as linear combinations of atomic orbitals by George Lisensky, Beloit College.
No2 Molecular orbital Diagram. molecular orbital theory structure of no2 pound the electron population of this orbital is see table vi 0 53 on the n atom 0 16 2s 0 37 2pz 0 24 on each o atom 0 24 pz experimentally oxides and oxyions of the non metals part ii c02 and no2 of the chemical society 1962 2873 2880 collects values for the partition of unpaired electron density among n and o atomic ...
According to molecular orbital theory, what is the bond order in the O2+ ion? Select one: a. 5.5 b. 5 c. 4 d. 2.5 e. 1.5 . The correct answer is: 2.5. Valence bond theory predicts that iodine will use _____ hybrid orbitals in ICl2-. Select one: a. sp2 b. sp3 c. sp3d d. sp3d2 e. None of these choices is correct. The correct answer is: sp3d. Which is the most reasonable prediction for the H-C-H ...
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
Organic Chemistry 4th ed - Paula Bruice
Answer to: What are the MO (molecular orbital) structures of (i) NO_2 and (ii) NO_2^-. (Include the sp^3, sigma, and all the non-bonding orbitals.)... for Teachers for Schools for Working Scholars ...
Nitrogen dioxide is a chemical compound with the formula NO 2.It is one of several nitrogen oxides. NO 2 is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the production of fertilizers.At higher temperatures it is a reddish-brown gas. It can be fatal if inhaled in large quantity.
In both molecules the pi symmetry molecular orbitals are the same. The 2p x orbitals on each atom combine to make a pi bonding and a pi antibonding molecular orbital in the xz plane. Perpendicular to these in the yz plane, the 2p y orbitals on each atom combine to make a pi bonding and a pi antibonding molecular orbital. Here is the full molecular orbital diagram for N 2.
You've seen the molecular orbital (MO) diagram of "CO"_2: "CO"_2 and "NO"_2^+ are isoelectronic and thus have the same electron configuration. Thus, simply add one or two electrons into the 2b_(3u) and 2b_(2u) to get "NO"_2 and "NO"_2^-, respectively. Nitrogen atom has 2p atomic orbitals lower by "2.52 eV", and 2s atomic orbitals lower by "6.13 eV" than with carbon atom.

















0 Response to "44 no2- molecular orbital diagram"
Post a Comment