42 how is activation energy represented on an energy diagram
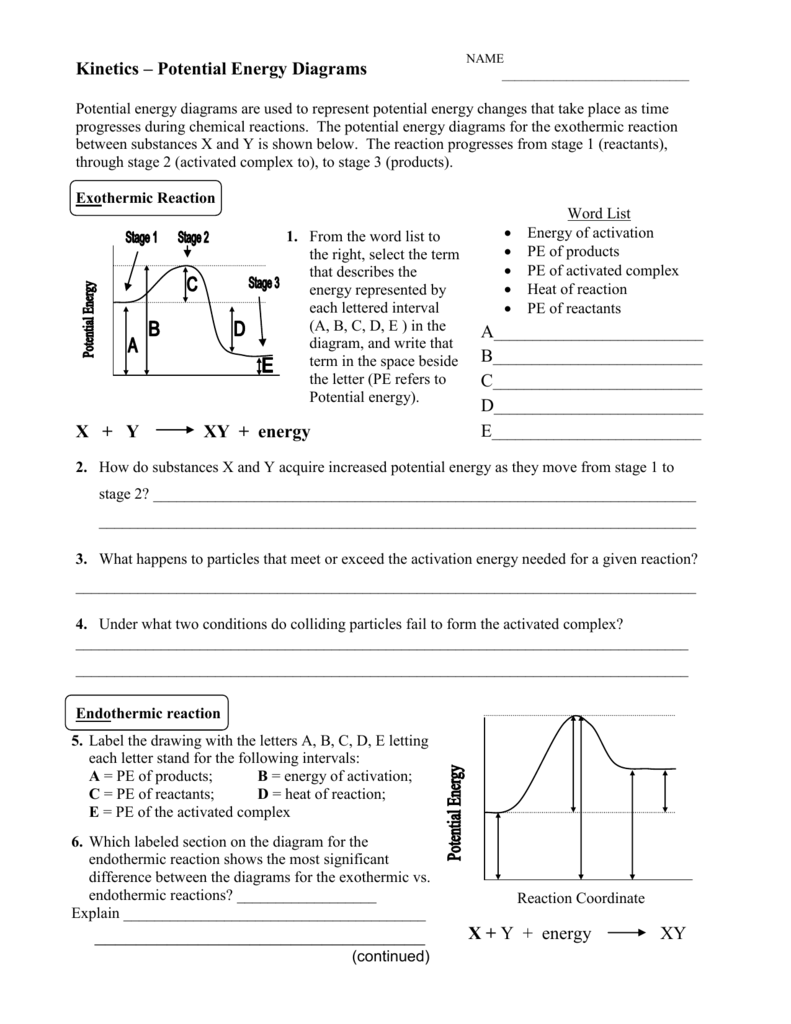
Potential Energy diagrams represent changes in the potential energy of the reacting particles forming products when they are colliding. Potential energy diagram worksheet answers 1. Label the axis PE of reactants 350 KJmol Ea 100 KJmol PE of products 250 KJmol. ... What is the value of the activation energy for the uncatalyzed reaction ... In accordance with the diagram, activation energy is the distance from the reactants to the top of the "hill"
The activation energy is represented by what on the diagram catalyzed reaction 3. Is this reaction shown on the diagram exothermic or endothermic 4. Ha catalyst was used on this reaction, explain what will be affected using the diagram S. Which represent the reactants and what is the energy of the reactants (Do not enter units)

How is activation energy represented on an energy diagram
In an energy diagram, the reaction progress is plotted on the x-axis and the potential energy is plotted on the y-axis. When the reaction begins the initial energy of the reactants is constant and then the energy starts increasing until maximum energy requirement is fulfilled. In the diagram of the changes in free energy during a chemical reaction, which term is best represented by the circled letter "C"? Activation energy When researchers analyzed the effects of temperature and reactant concentration on reaction rates (see figure), why were temperature and concentration examined separately? Activation energy, transition state, and reaction rate. ... The activation energy shown in the diagram below is for the forward reaction (reactants ...
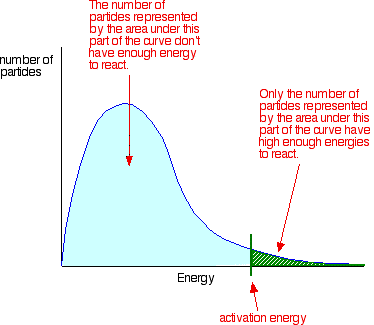
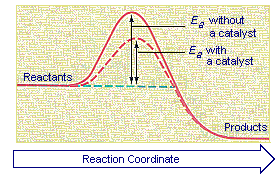
How is activation energy represented on an energy diagram. D) 3.49 x 104 years- E) 3.97 x 103 years- . Consider the reaction coordinate diagram shown below. The activation energy for the forward reaction is best represented by which number shown in the diagram? reaction pathway A) 1 B) 2 C) 3 D) 4 E) 5 re to search a 11:17 AM 2/18/202 potential energy In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. Which line in the diagram represents the activation energy for a forward reaction? Learn this topic by watching Energy Diagram Concept Videos All Chemistry Practice Problems Energy Diagram Practice Problems See all problems in Energy Diagram Frequently Asked Questions What scientific concept do you need to know in order to solve this problem? Changes in activation energy during a chemical reaction are represented by a. potential energy diagram. One change in a reaction, other than adding a catalyst, that can increase the rate of the given reaction. is a change in temperature.
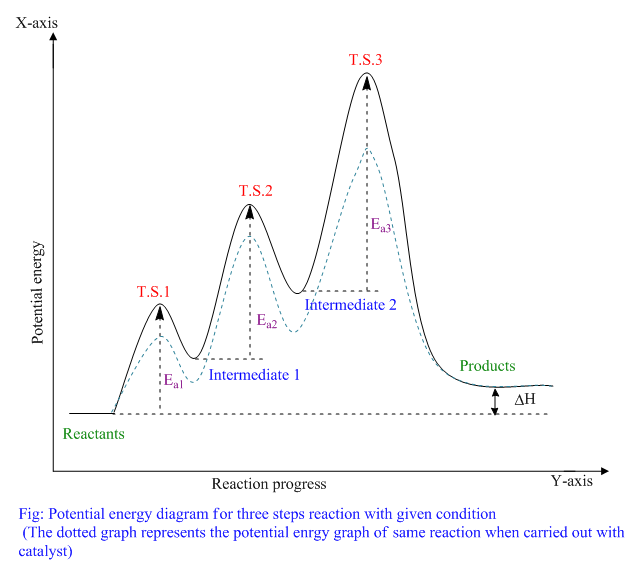
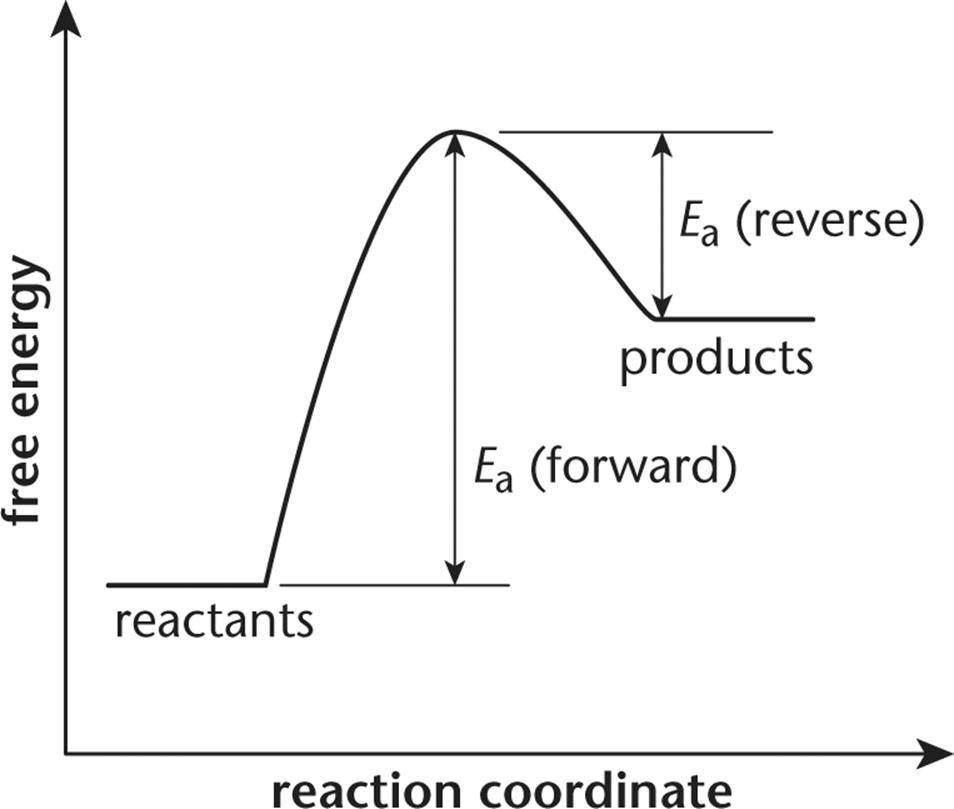
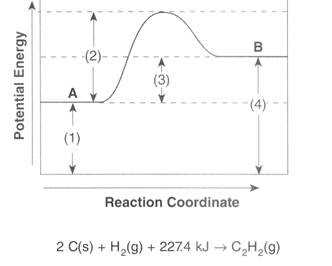
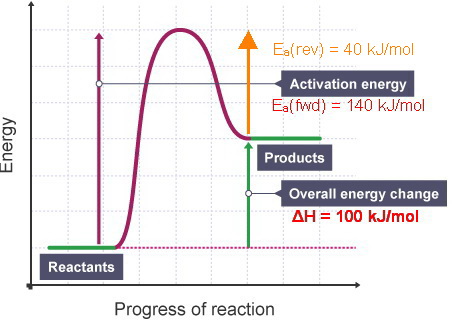
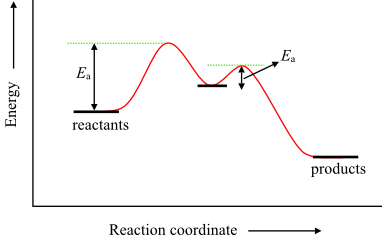
To find the activation energy, you should be looking for two numbers: the potential energy of the reactants and the energy of the activated complex (the maximum point). (energy of activation complex) - (PEreactants) (100 kJ) - (40 kJ) = 60 kJ. In other words, it takes 60 kJ of energy to complete the reaction. diagram. The activation energy for the reverse reaction, E a(rev), is the difference between the product energy and transition state at the peak of the diagram. ... Draw a potential energy diagram that would reasonably represent this combustion reaction. The activation energy for the reverse reaction is represented by A) 1 and 3 B) 3 and 4 C)2 and 3 D) 1 and 2 12.The potential energy diagram below shows the reaction X + Y Z. When a catalyst is added to the reaction, it will change the value of A) A B)B C) C D) D 13.In the potential energy diagram below, which letter 9. The accompanying diagram represents the energy changes that occur during the formation of a certain compound under standard conditions. According to Reference Table G, the compound could be A. C2H6(g) B. CO2(g) C. ICl(g) D. SO2(g) 10. A potential energy diagram is shown. Which letters represent the activation energy of the forward and
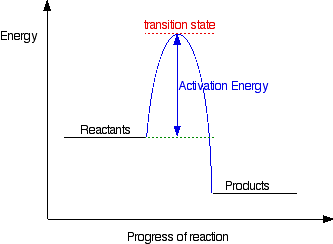
Purists might also note that the symbol used to represent the activation energy is written with a capital " E ". This is unfortunate, because it leads students to believe the activation energy is the change in the internal energy of the system, which is not quite true. Jun 26, 2015 · Label the vertical axis "Potential Energy" and the horizontal axis "Reaction Coordinate". 2. Draw and label two short horizontal lines to mark the energies of the reactants and products. 3. Draw the energy level diagram. There must be a hump in the curve to represent the energy level of the activated complex. 4. Draw and label the activation energy. The energy of the reactants increase and then decrease to the final product energy. The highest point in the curve represents the energy of the intermediate state in the reaction. The energy... Typically, we envision reactions proceeding left to right along the reaction coordinate, so often, the activation energy is only noted for the forward reaction. The activation energy on the diagram below shows the barrier to be 102.6 kJ mol -1. Barriers are measured in energy per mole (typically kJ mol -1 ). Arrhenius Law and Temperature Dependence
Feb 23, 2020 · For a forward reaction, the activation energy is equal to the difference between the threshold energy and the energy level of the reactants. Once you identify the threshold energy and the energy level of the reactants, use a double arrowhead line to connect these two points on the potential energy diagram.
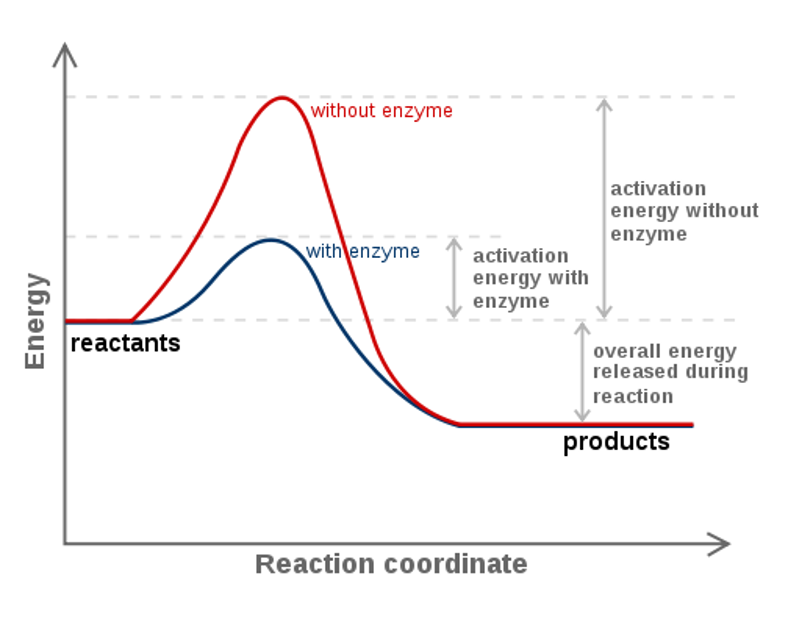
The energy diagram would look something like this. So the activation energy for the reverse reaction is the sum of the enthalpy (delta H) and theactivation energy (Eact) for the forward reaction. Note that the enthalphy change is negative for the forward reaction. It means that energyis released from the reaction 11.5K views View upvotes
Activation energy is usually represented by E a and find from the Arrhenius mathematical formula, k = Ae -Ea/RT, where A = constant. It is obvious that the E a in the Arrhenius equation must have the units of energy. Generally, it is measured by the unit like joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol).
For a forward reaction, the activation energy is equal to the difference between the threshold energy and the energy level of the reactants. Once you identify the threshold energy and the energy level of the reactants, use a double arrowhead line to connect these two points on the potential energy diagram.
Activation energy in chemistry is the amount of energy required to start a reaction. For every chemical reaction, a certain amount of energy is required to start it. The diagram represents how much...
Dec 19, 2021 · Activation energy is the energy required for a reaction to proceed. In a diagram activation energy is graphed as the height of an energy barrier between two minimum points of potential energy. The activation energy Ea is the difference between the energy of the activated complex and that of the reactants. 11500 Jmol 23 kJmol X 1000 34500 Jmol.
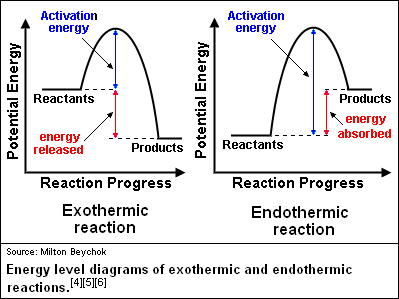
The activation energy is the energy required to break bonds so that a reaction can start. Energy changes during a reaction can be displayed in an energy profile diagram. Exothermic reactions When the activation energy is less than the energy released when the “new” bonds form there is an overall release of energy, (usually as heat released ...
the activation energy (ex) is represented by letter a potential energy d e reaction coordinate question 20 (1 point) refering to the energy diagram below, the reaction shown in the diagram is a · (exothermic or endothermic) potential energy d e reaction coordinate question 21 (1 point) referring to the two energy diagrams shown below (assuming …
Activation energy, transition state, and reaction rate. ... The activation energy shown in the diagram below is for the forward reaction (reactants ...
In the diagram of the changes in free energy during a chemical reaction, which term is best represented by the circled letter "C"? Activation energy When researchers analyzed the effects of temperature and reactant concentration on reaction rates (see figure), why were temperature and concentration examined separately?
In an energy diagram, the reaction progress is plotted on the x-axis and the potential energy is plotted on the y-axis. When the reaction begins the initial energy of the reactants is constant and then the energy starts increasing until maximum energy requirement is fulfilled.



















0 Response to "42 how is activation energy represented on an energy diagram"
Post a Comment