44 diagram of energy states and transitions in the hydrogen atom
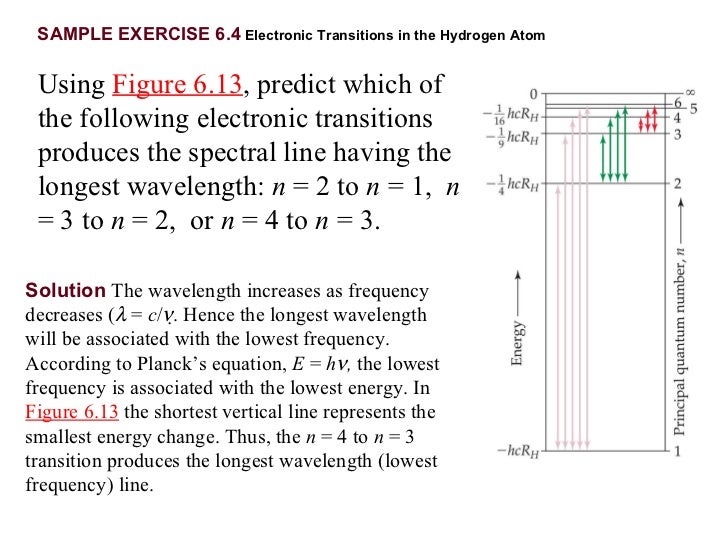
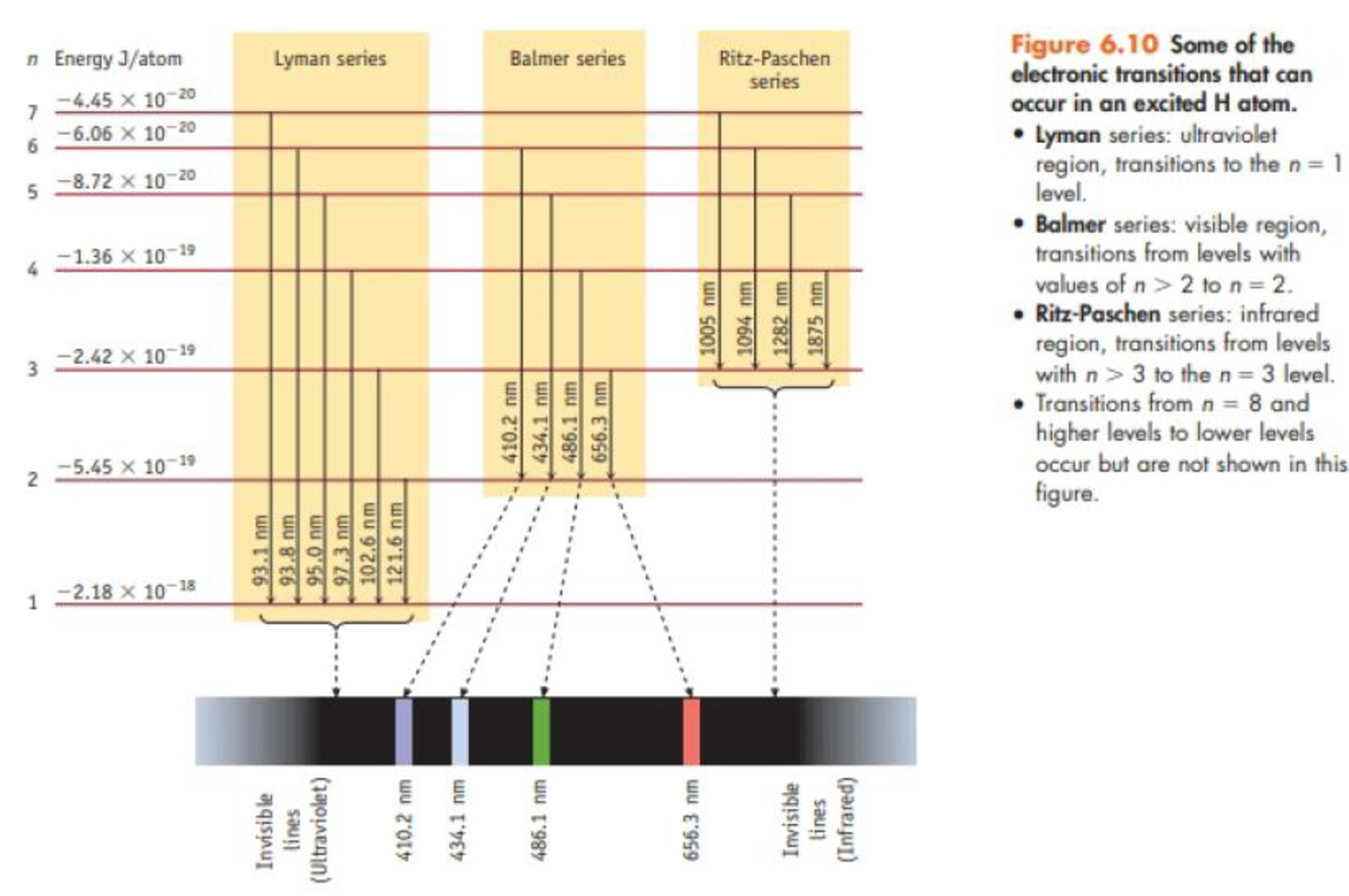
What are the degeneracies of the Hydrogen atom energy levels? Recall they are dependent on the principle quantum number only. III. Spectroscopy of the Hydrogen Atom Transitions between the energy states (levels) of individual atoms give rise to characteristic atomic spectra. These spectra can be used as analytical tools to assess composition of ... 3 state, the maximum energy of the atom is −13.6 eV+12.3=1.3. From the energy level diagram, Figure 37-26, we see that this means the atom could be excited to the n = 3 state, so the possible transitions when the atom returns to the ground state are n = 3 to n = 2, n = 3 to n = 1, and n = 2 to n = 1. We calculate the wavelengths from the equation
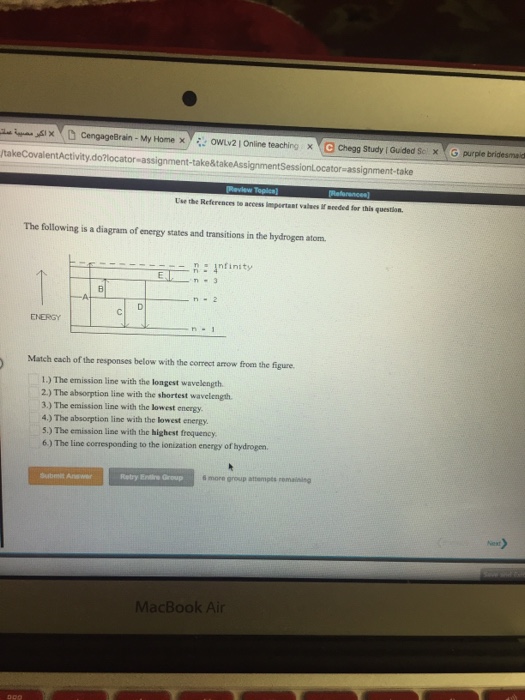
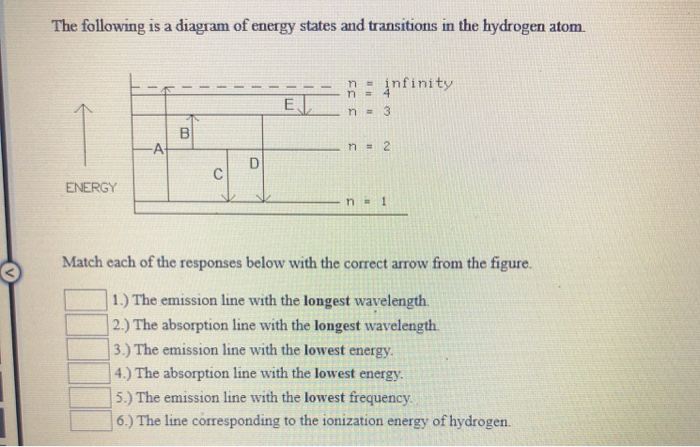
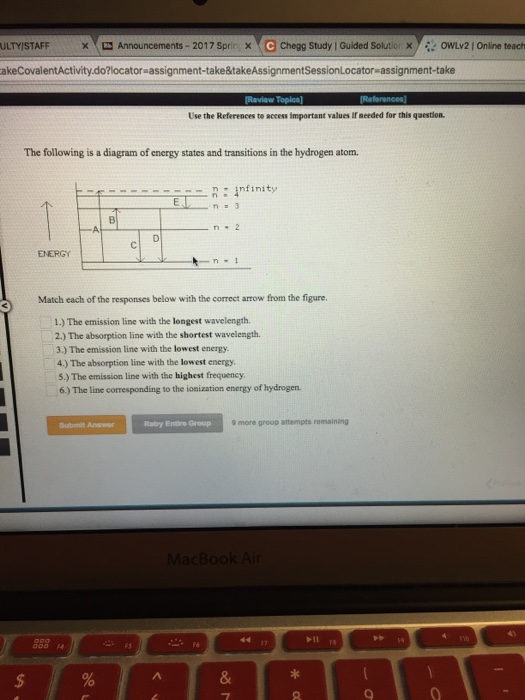
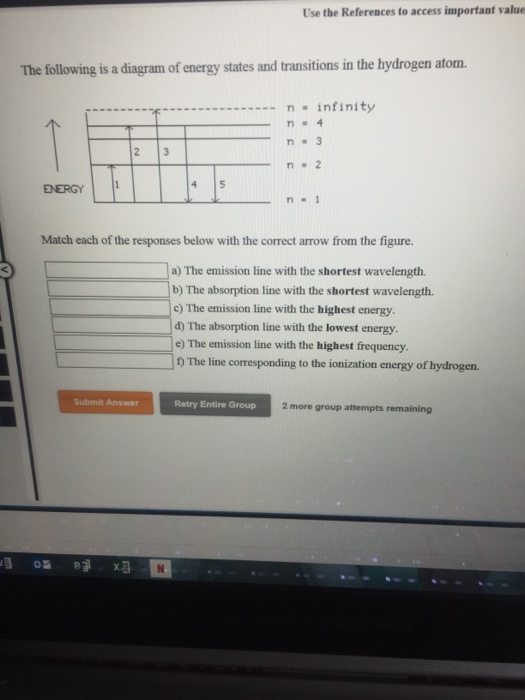
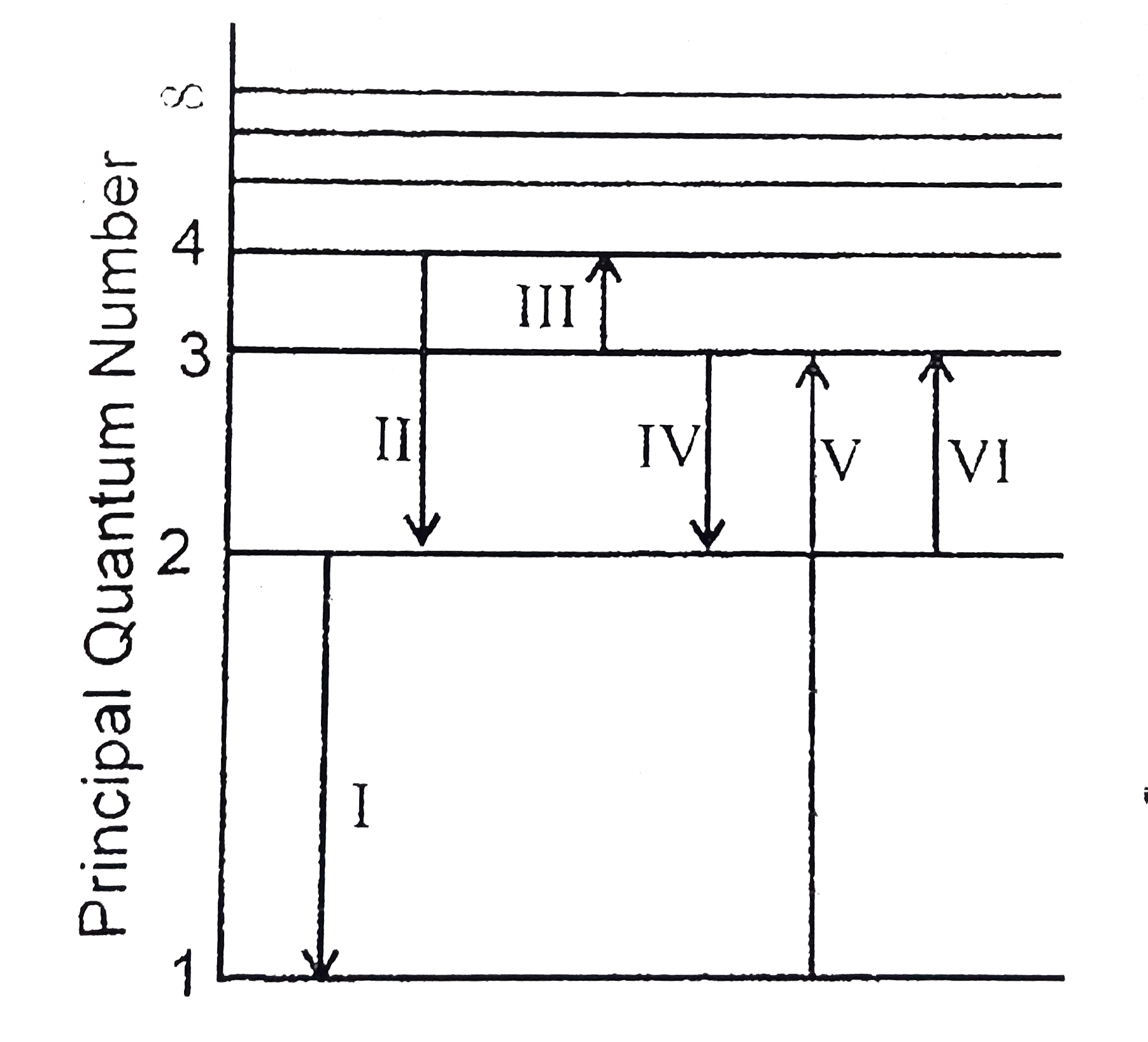
Question: The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the figure. a) The emission line with the ...
Diagram of energy states and transitions in the hydrogen atom
the energy of the light absorbed when an electron in . a hydrogen. atom. makes the . following transitions: (a) n = 1 to n = 4; (b) n = 3 to n = 8; (c) n = 2 to n = 4. Give. your . answers . to. 3 significant . figures. (d) Draw an energy level diagram for hydrogen, and . draw vertical arrows to indicate each of these three electron transitions ... The shift of the energy levels of an atom due to a magnetic field is called the Zeeman effect. We say that the curves in Fig. 12-3 show the Zeeman splitting of the ground state of hydrogen. When there is no magnetic field, we get just one spectral line from the hyperfine structure of hydrogen. Draw a neat labelled diagram showing energy levels and transition between them for the hydrogen atoms. - Physics. ... Energy levels and transition between them for hydrogen atom: Concept: Structure of Atoms and Nuclei ... Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Commerce;
Diagram of energy states and transitions in the hydrogen atom. This modification of the energy levels of a hydrogen atom due to a combination of relativity and spin-orbit coupling is known as fine structure.. Now, it is conventional to refer to the energy eigenstates of a hydrogen atom which are also simultaneous eigenstates of as states, where is the radial quantum number, as , and is the total angular momentum quantum number. The following is a diagram of energy states and transitions in the hydrogen atom. Match each arrow with the correct response below. a) The emission line with the longest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the lowest energy. d) The absorption line with the highest energy. e) The emission line with the highest frequency. The 3 d to 2 p transition is a transition from a state with quantum numbers n = 3, l = 2 to a state with n = 2, l = 1. The change in m has to be -1, or 0 or +1. The diagram shows the energy level splitting and the allowed transitions. The different colored arrows show the 3 different photon energies. (c) Because the additional energy due to ... The following is a diagram of energy states and transitions in the hydrogen atom. Match each arrow with the correct response below. The emission line with the longest wavelength. The absorption line with the longest wavelength. The emission line with the highest energy. The absorption line with the highest energy.
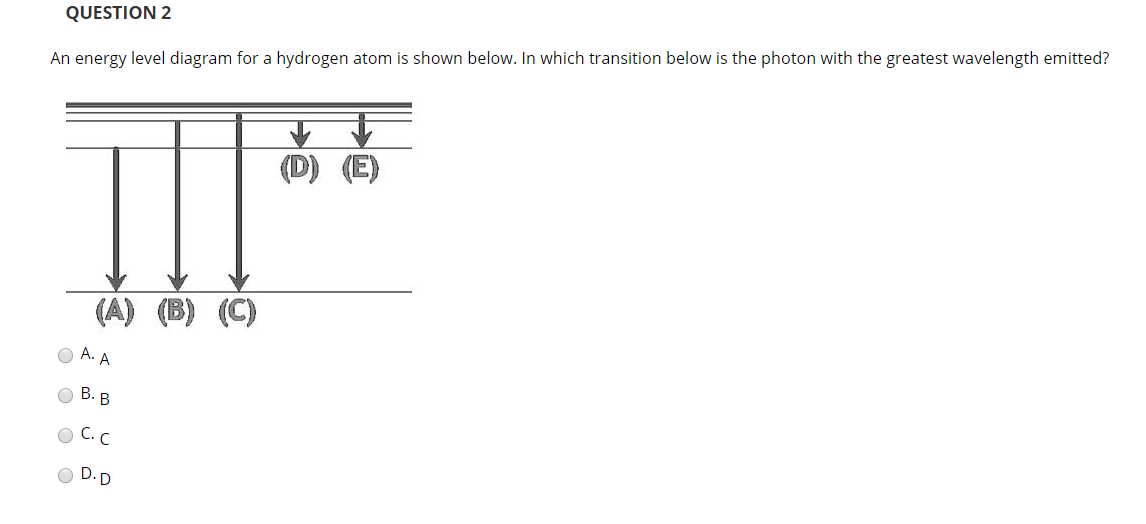
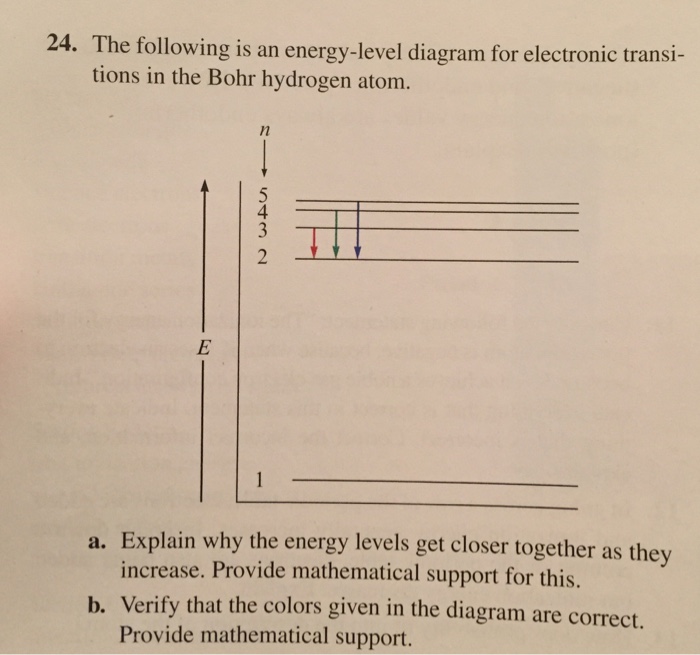
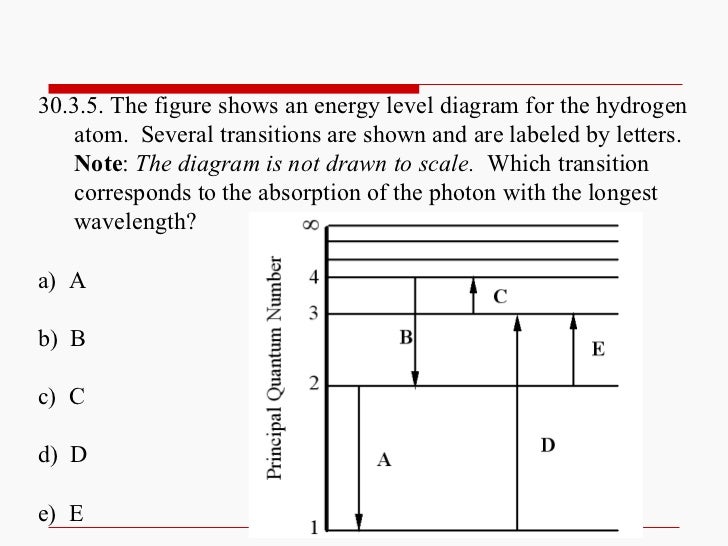
22) The diagram represents energy levels in a hydrogen atom. The labeled transitions (A through E) represent an electron moving between energy levels. Suppose that an electron in a hydrogen atom absorbs 10.2 eV of energy, so that it moves from level 1 to level 2. Electron Transitions The Bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy: This is often expressed in terms of the inverse wavelength or "wave number" as follows: The reason for the variation of R is that for hydrogen the mass of the orbiting electron is not negligible compared to ... Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from a particular state to a lower state, it is losing energy. Transitions in Hydrogen. Let us calculate the rate of spontaneous emission between the first excited state ( i.e., ) and the ground-state ( i.e., ) of a hydrogen atom. Now the ground-state is characterized by . Hence, in order to satisfy the selection rules ( 1149) and ( 1150 ), the excited state must have the quantum numbers and .
Refer to the energy level diagram for the transitions of the hydrogen atom in your lab manual to answer the following: What is the energy of the principal quantum number 3, in units of reciprocal centimetres, cm -1? What principal quantum number has an energy of -6855 cm. -1. Referring to the two values above, what amount of energy is given off ... The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the figure. Learn this topic by watching Bohr Model Concept Videos. All Chemistry Practice Problems Bohr Model Practice Problems. See all problems in Bohr Model. The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon. For the hydrogen atom, the energy levels only depend on the principal quantum number n. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy.
This states that the energy scale of hydrogen bound states is a factor of α2 smaller than the rest energy of the electron, that is, about 19000 times smaller. We can thus rewrite the possible energies as: En = −1 2α 2 mc2 1 n. (2.1.6) The typical momentum in the hydrogen atom is p≃ ~ a 0 = me2 ~ = e2 ~c mc → p≃ α(mc), (2.1.7) which, written as p≃ m(αc) says that the typical velocity is v≃ αc, which is low enough
The following is a diagram of energy states and transitions in the hydrogen atom. n = n infinity EL n = 3 B -A n = 2 D rogress n = 1 Match each of the responses below with the correct arrow from the figure. 1.) The emission line with the longest wavelength. 2.) The absorption line with the longest wavelength. 3.)
The problem is that hydrogen atoms are hit with electrons with a certain energy, and I am to find what energy levels the electrons og the atom can be exited into. The energy of the electrons hitting the atom is 2.072 aJ. We have learned to find wavelengths and such things. But, as far as I know we haven't gone through a problem like this. And the relevant chapter in the books doesn't quite explain how to go about this problem.
The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the figure. 1.) The emission line with the longest wavelength._____ 2.) The absorption line with the longest wavelength.____ 3.) The emission line with the lowest energy._____ 4.)
The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the figure. a) The emission line with the longest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the lowest energy. d) The absorption line with the highest ...
The following is a diagram of energy states and transitions in the hydrogen atom. n = infinity 4 n = 3 3 n = 2 ENERGY 4 n = 1 Match each arrow with the correct response below. a) The emission line with the shortest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the lowest energy.
The following is a diagram of energy states and transitions in the hydrogen atom. infinity %3D 4 3 4 ENERGY Match each of the responses below with the correct arrow from the figure. a) The emission line with the shortest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the lowest energy.

SAKURAI - THE MIND-BLOWING CAPTURE OF AN ELECTRON OBITAL --- THE NEW HIGH-ENERGY IMAGES AT CERN OF A HELIUM ANTIMATTER / MATTER NUCLEON IN REAL-TIME AND CAPTURED AT THE SPEED OF LIGHT.
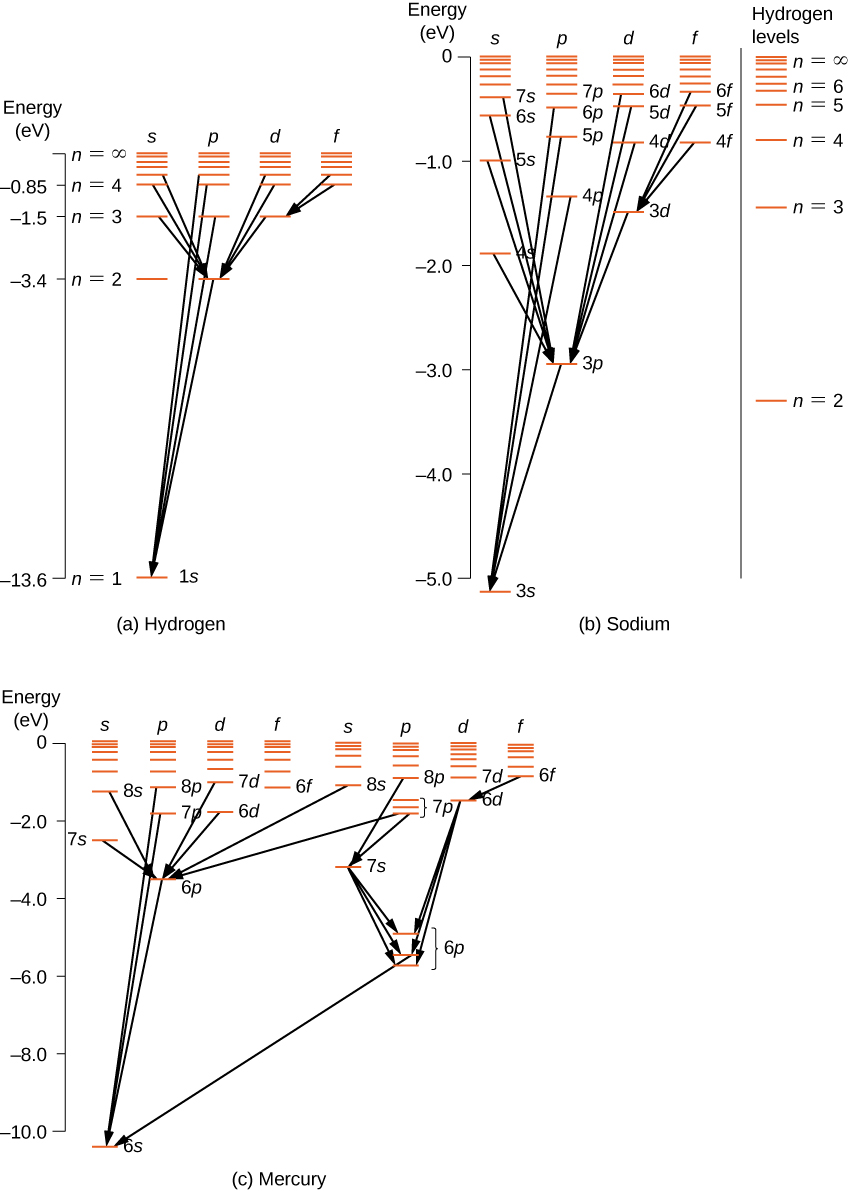
Hydrogen atom quantum numbers A Grotrian diagram is what spectroscopists use to analyze their line spectra. Each column is for a different l quantum number. Note that only certain transitions are observed. These are called selection rules. s p d f g h angular momentum quanta l principal quanta n
An electron in a hydrogen atom is in an excited state of 3d transitions radiatively to the 2p energy level, without regarding the spin-orbit interaction: a. Show the energy level splitting of 3d and 2p if there's no magnetic field and with magnetic field B in one energy level diagram. b.
To discuss energy shifts for particular states, it is helpful to introduce some nomenclature from atomic physics. For a state with principal quantum number n, total spin s, orbital angular momentum #, and total angular momentum j, one may define the state by the spectroscopic notation, n 2s+1L j For a hydrogen-like atom, with just a single ...
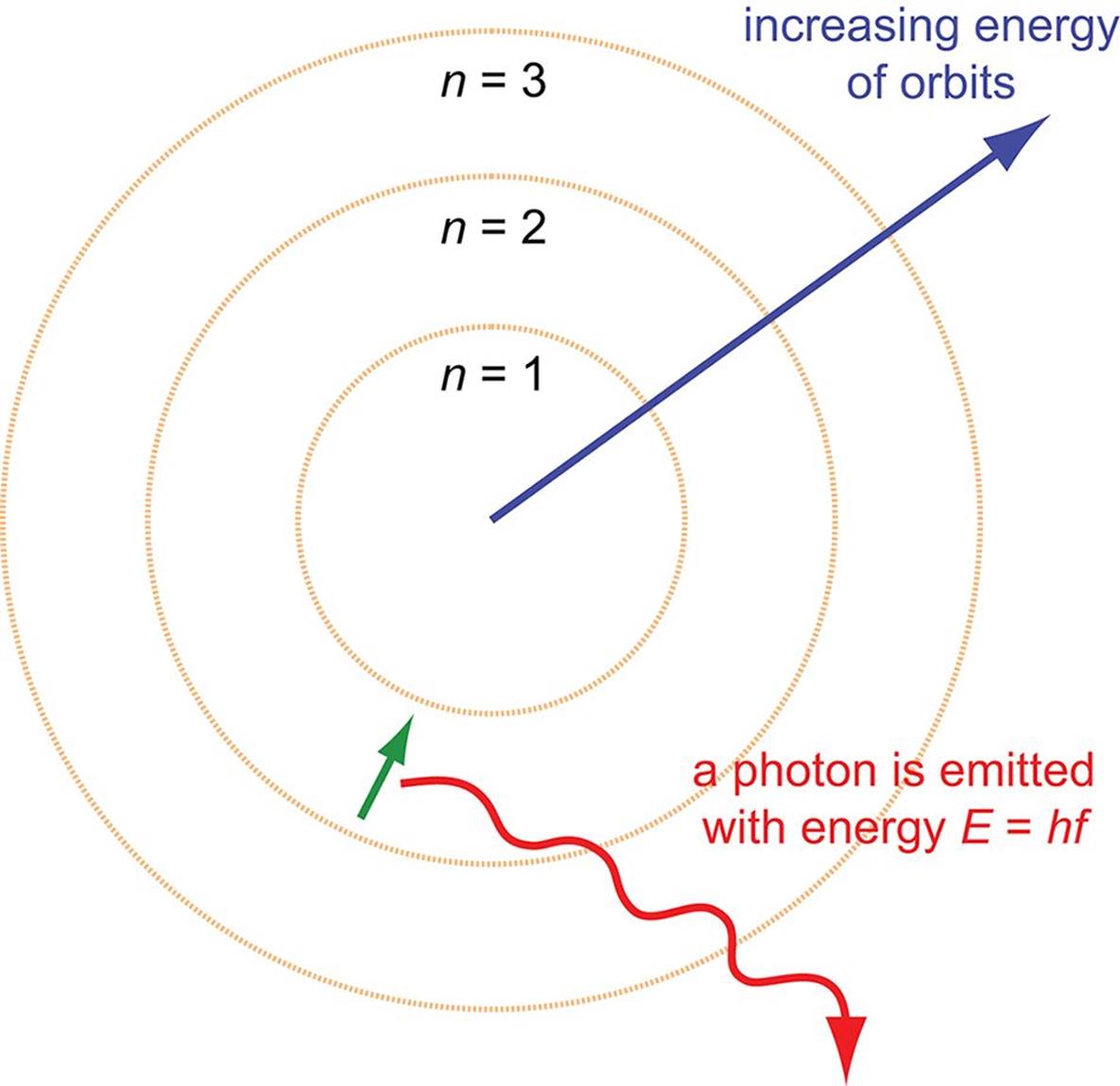
Bohr's model of the hydrogen atom explains the emission and absorption spectra of atomic hydrogen and hydrogen-like ions with low atomic numbers. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom.
In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. According to Bohr's theory, electrons of an atom revolve around the nucleus on certain orbits, or electron shells. Each orbit has its specific energy level, which is expressed as a negative value. This is because the electrons on the orbit are "captured ...
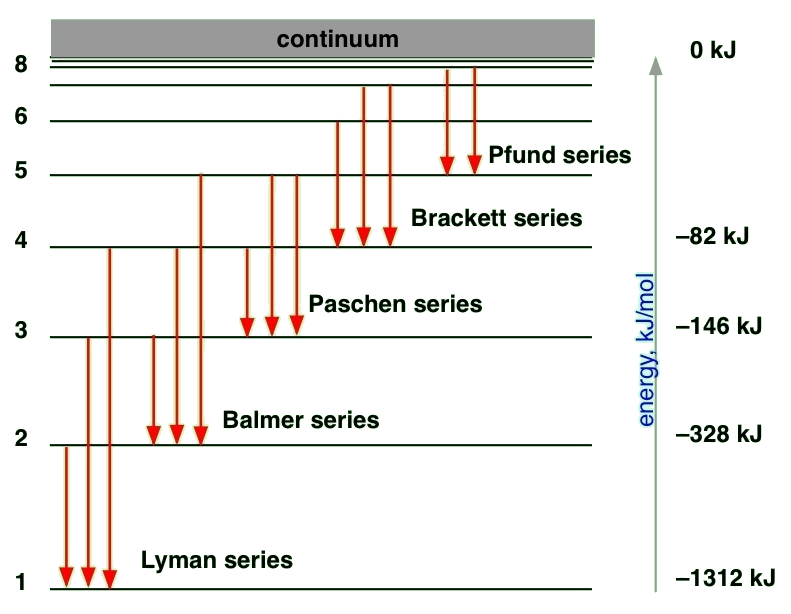
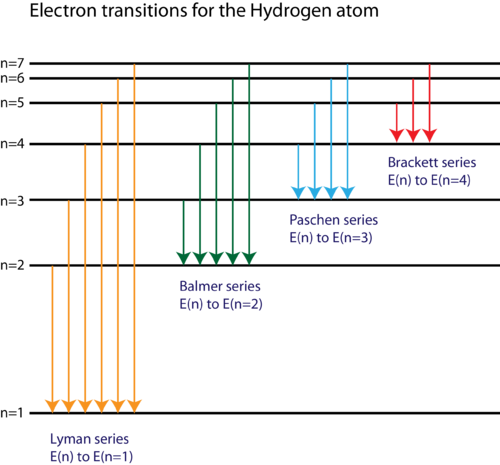
Electron transitions and their resulting wavelengths for hydrogen Three of the transition series in hydrogen (not to scale). The emission spectrum of hydrogen Some of the most common and readily observable series have been named as shown in this image, where n 1 is the ground state and n 2 are excited states.
Draw a neat labelled diagram showing energy levels and transition between them for the hydrogen atoms. - Physics. ... Energy levels and transition between them for hydrogen atom: Concept: Structure of Atoms and Nuclei ... Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Commerce;
The shift of the energy levels of an atom due to a magnetic field is called the Zeeman effect. We say that the curves in Fig. 12-3 show the Zeeman splitting of the ground state of hydrogen. When there is no magnetic field, we get just one spectral line from the hyperfine structure of hydrogen.
the energy of the light absorbed when an electron in . a hydrogen. atom. makes the . following transitions: (a) n = 1 to n = 4; (b) n = 3 to n = 8; (c) n = 2 to n = 4. Give. your . answers . to. 3 significant . figures. (d) Draw an energy level diagram for hydrogen, and . draw vertical arrows to indicate each of these three electron transitions ...















0 Response to "44 diagram of energy states and transitions in the hydrogen atom"
Post a Comment