45 pb sn phase diagram
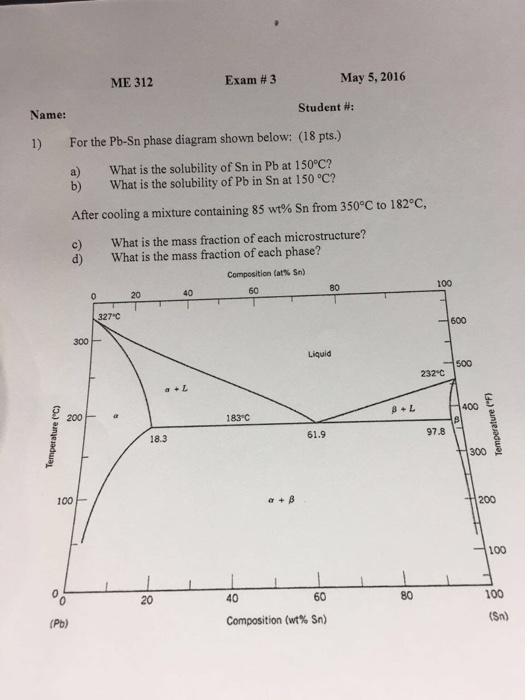
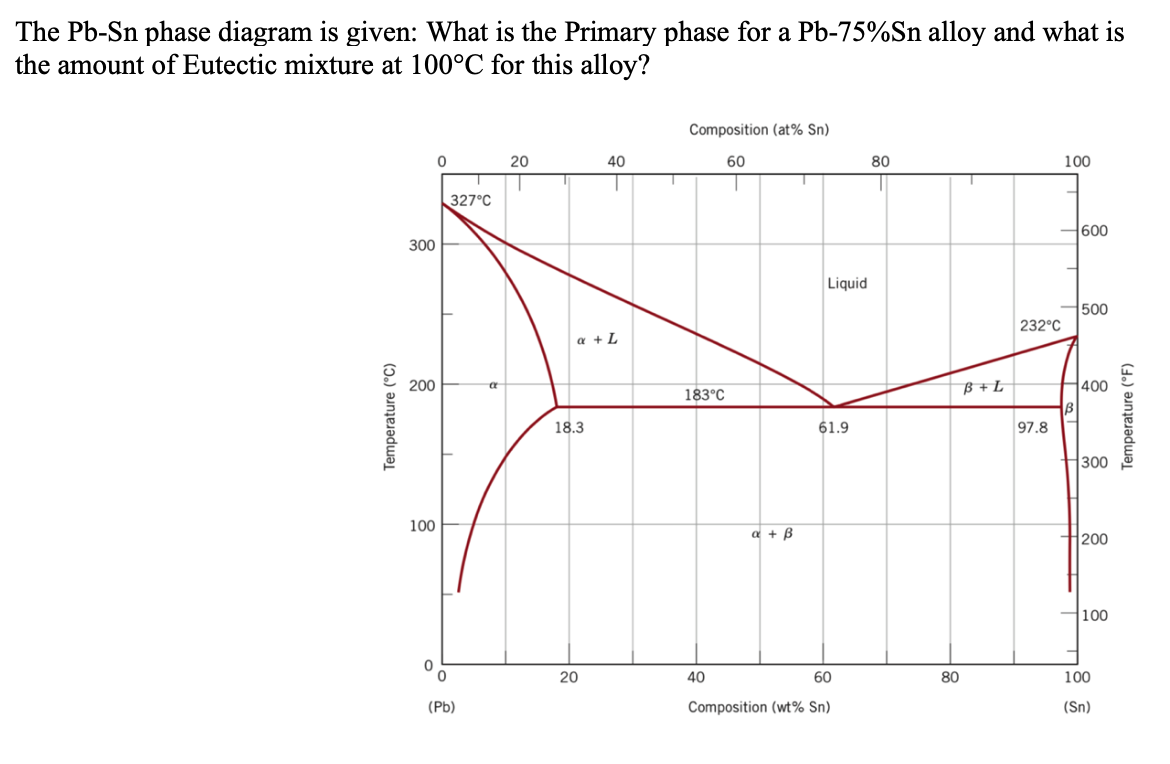
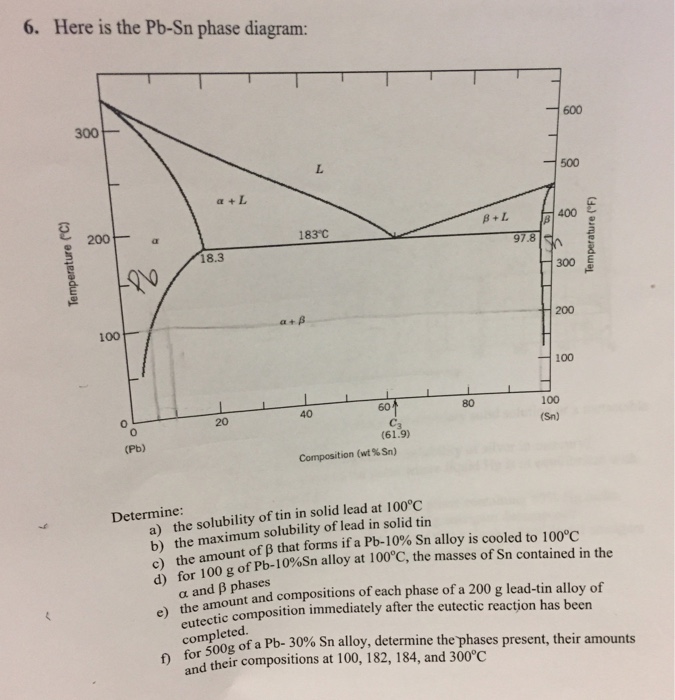
The lead-tin phase diagram is shown below. Round to the nearest whole number. This problem has been solved! See the answer See the answer See the answer done loading. At 100 C, what is the maximum solubility (a) of Pb in Sn? (b) of Sn in Pb? The lead-tin phase diagram is shown below. Round to the nearest whole number. represented in the portion of the Pb-Sn phase diagram shown below (at point B). Furthermore, the compositions of the phases, as determined from the tie line are Cα = 16 wt% Sn-84 wt% Pb Cβ = 97 wt% Sn-3 wt% Pb Inasmuch as the composition of the alloy C0 = 75 wt% Sn, application of the appropriate lever rule expressions (for
12 Figure 9.2 The copper-nickel phase diagram . 3. ปริมาณหรือสัดส่วนของ Phase ที่ปรากฏอยู่ (Phase Amounts) 2. ... โลหะผสมหนึ่งในระบบ Pb-Sn มีเฟส ...
Pb sn phase diagram
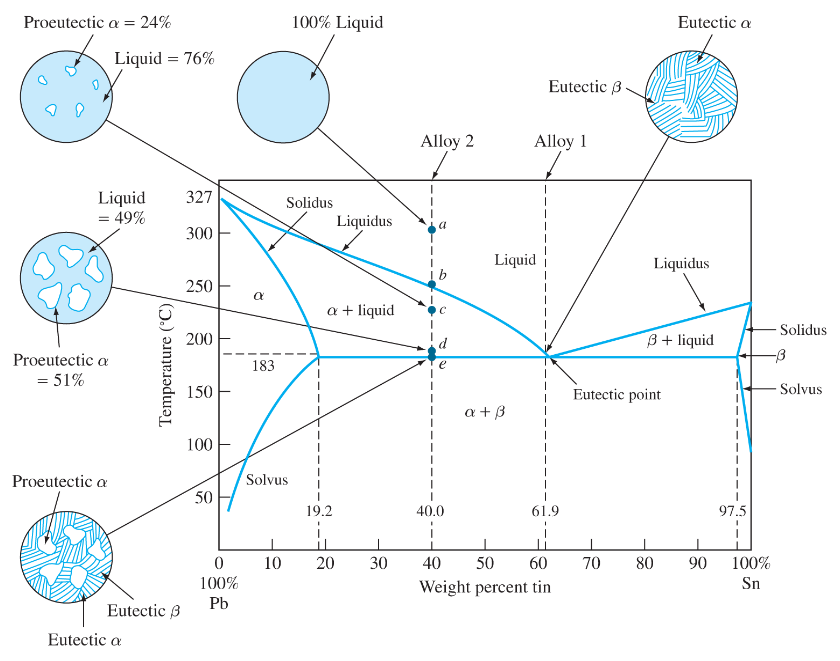
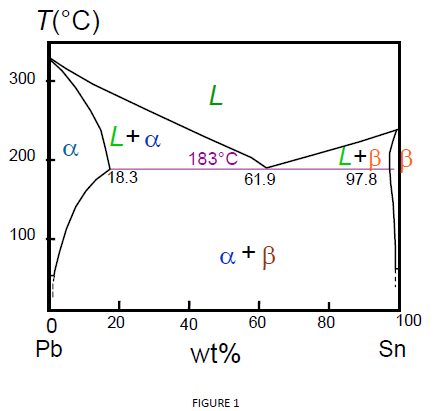
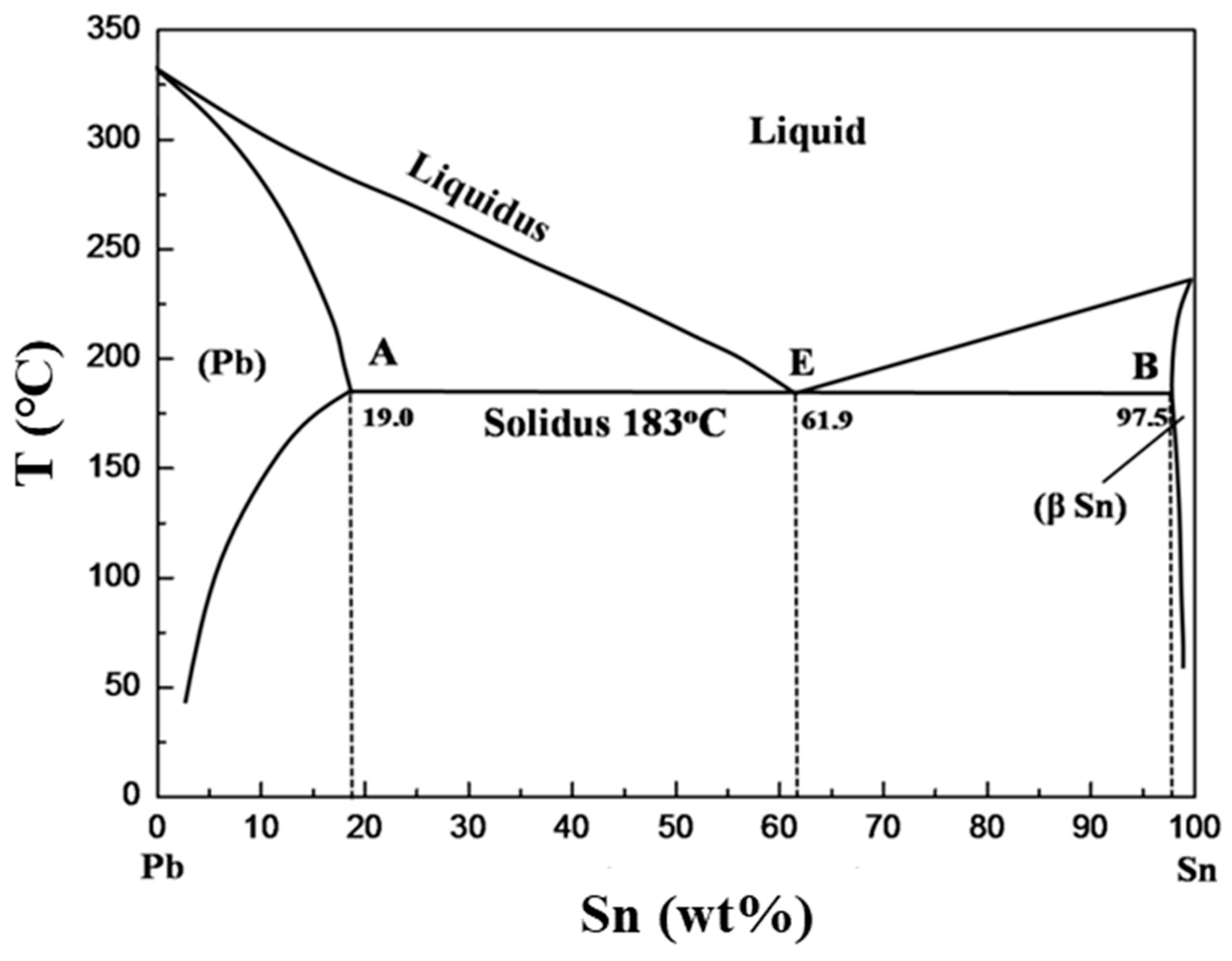
(b) That portion of the Pb-Sn phase diagram (Figure 9.8) that pertains to this problem is shown below; the point labeled “B” represents the 75 wt% Sn-25 wt% Pb composition at 175°C. As may be noted, point B lies within the α + β phase field. A tie line has been constructed at 175°C; its intersection Pb-Sn phase diagram β phase: solid solution of Pb in tetragonal Sn α phase: solid solution of Sn in fcc Pb Liquid Pb (Fcc) Sn (Tetra) 0 50 100 150 200 250 300 350 0 10 20 30 40 50 60 70 80 90 100 T emperature Wt% The Pb-Sn system is characteristic of a valley in the middle. Such system is known as the Eutectic system. The central point is the Eutectic Pb-Sn Phase Diagram Liquidus Solidus Solidus Solidus Solvus Solvus 28. 28 Solidification of Eutectic Mixtures • Mixtures of some metals, such as copper & nickel, are completely soluble in both liquid and solid states for all concentrations of both metals. Copper & nickel have the same crystal structure (FCC) and have nearly the same atomic radii.
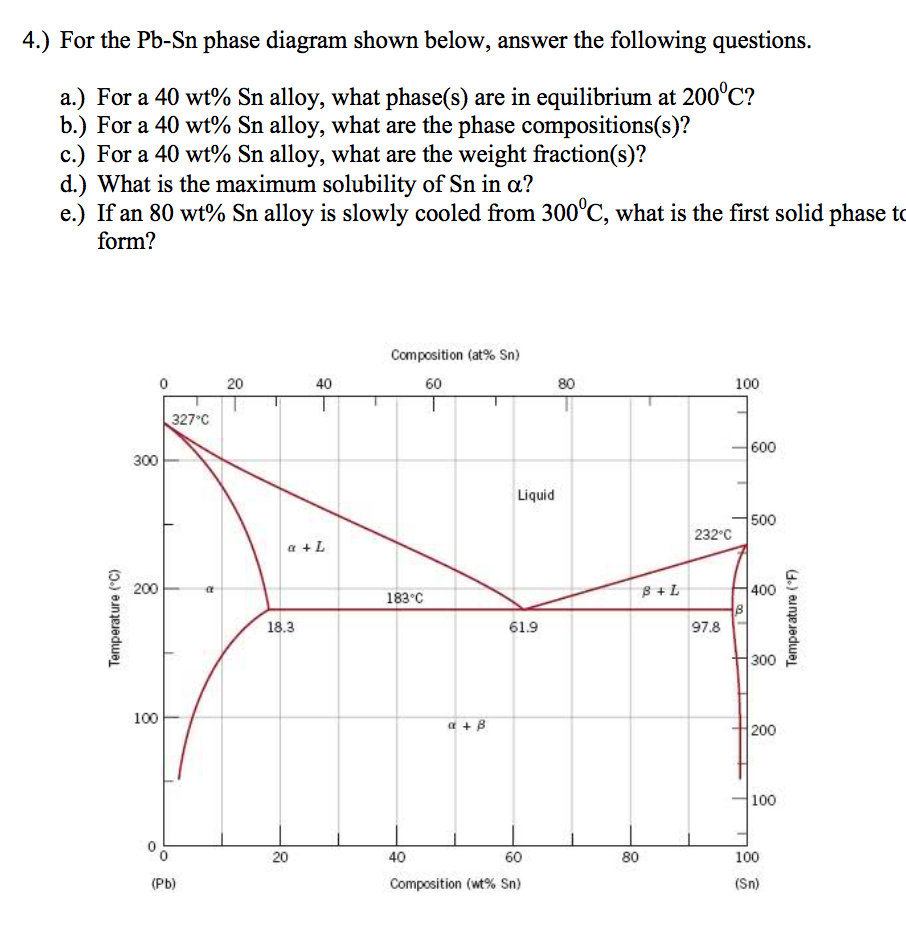
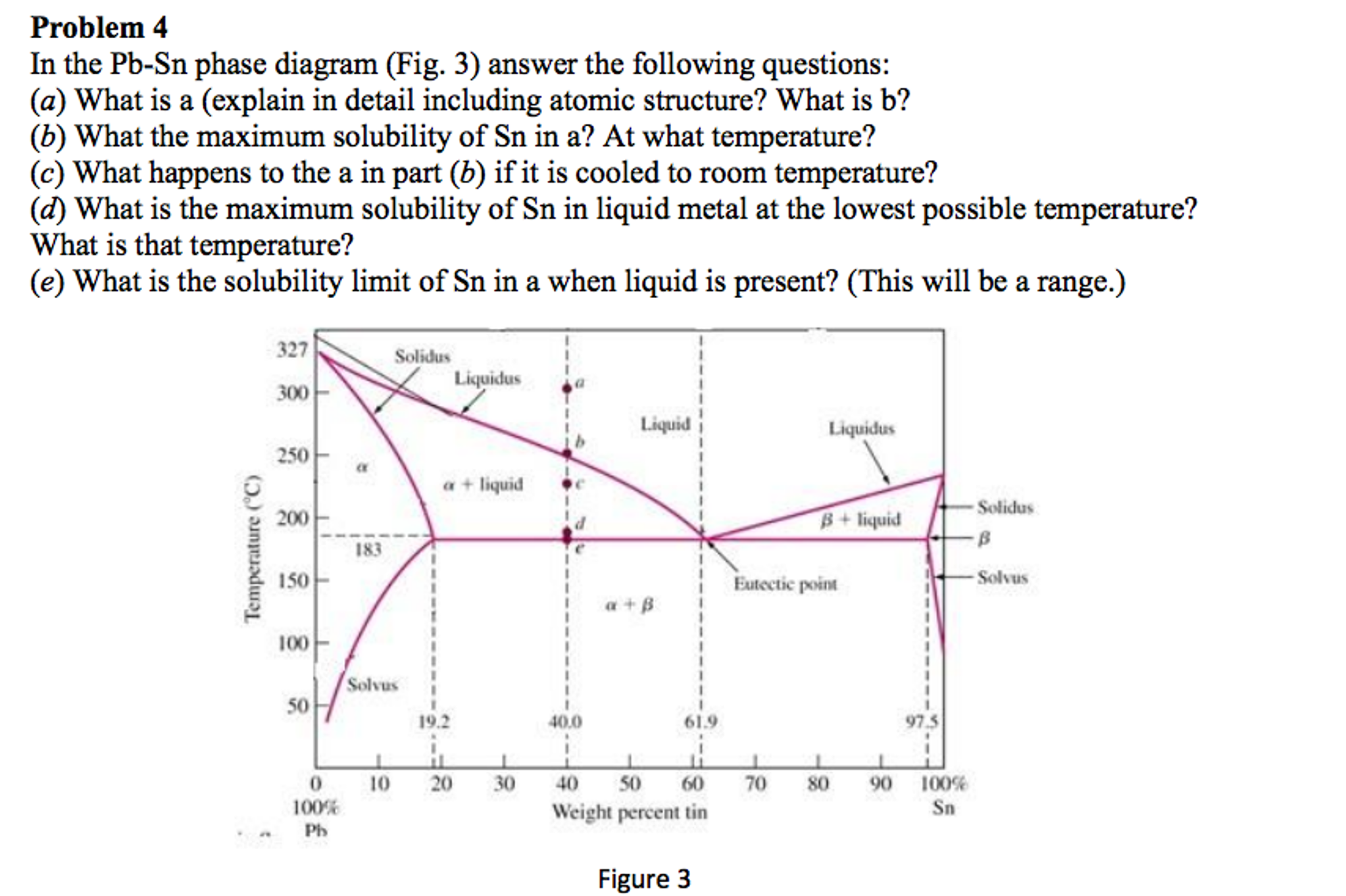
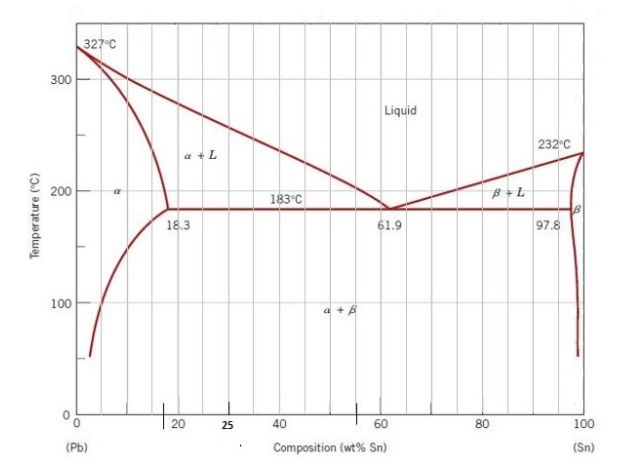
Pb sn phase diagram. This video explains the Pb-Sn phase diagramFor further reading: https://www.physicsforums.com/threads/sn-pb-phase-diagram.281790/ Created Date: 11/3/2015 10:28:58 AM Phase Analysis from Sn-Pb Phase Diagram 3. Question: For a 40-60 Pb-Sn solder, find ; a) Phase present, Composition of phases and Weight fraction at 200˚C b) Phase present, Composition of phases and Weight fraction at 100˚C 4. Phase Diagram: 5. In the Pb-Sn phase diagram below, there are 6 phase fields: three shaded purple and three shaded white. The three purple phases fields are single phase regions, regions in which only one phase exists, and the white phase fields are two-phase regions.
The (Sn) phase is the primary phase in all cases. Under Scheil conditions the alloy Sn-.04Bi-.06Pb encounters the L -> (Sn) + (Pb) monovariant eutectic where both phases form simultaneously from the liquid phase. The path encounters then the four phase reaction, L + (Pb) -> (Sn) + epsilon. Under Scheil assumptions fraction and concentration ... Important: This is a simplified version of the real tin-lead phase diagram.In particular, it ignores the formation of solid solutions of tin and lead. You will find the correct diagram on this NIST web page.Beware that on that page, the tin-lead axis is reversed from the one I have drawn above - in other words 100% lead is on the right rather than the left. 9.38 On the basis of the photomicrograph (i.e., the relative amounts of the microconstituents) for the lead- tin alloy shown in Figure 9.17 and the Pb-Sn phase diagram (Figure 9.8), estimate the composition of the alloy, and then compare this estimate with the composition given in the figure legend of Figure 9.17. Phase Struktur-bericht Symbol Common Names Prototype Spacegroup Model * Liquid: n/a: L: n/a: n/a (Bi,Pb,Sn) 1 : Fcc: A1 (Pb) Cu: Fm-3m (Bi,Pb,Sn) 1 (Va) 1: Hcp: A3 (epsilon Pb) Mg: P6 3 /mmc (Bi,Pb,Sn) 1 (Va) 0.5: Rho: A7 (Bi) alpha As: R-3m (Bi,Pb,Sn) 1 *
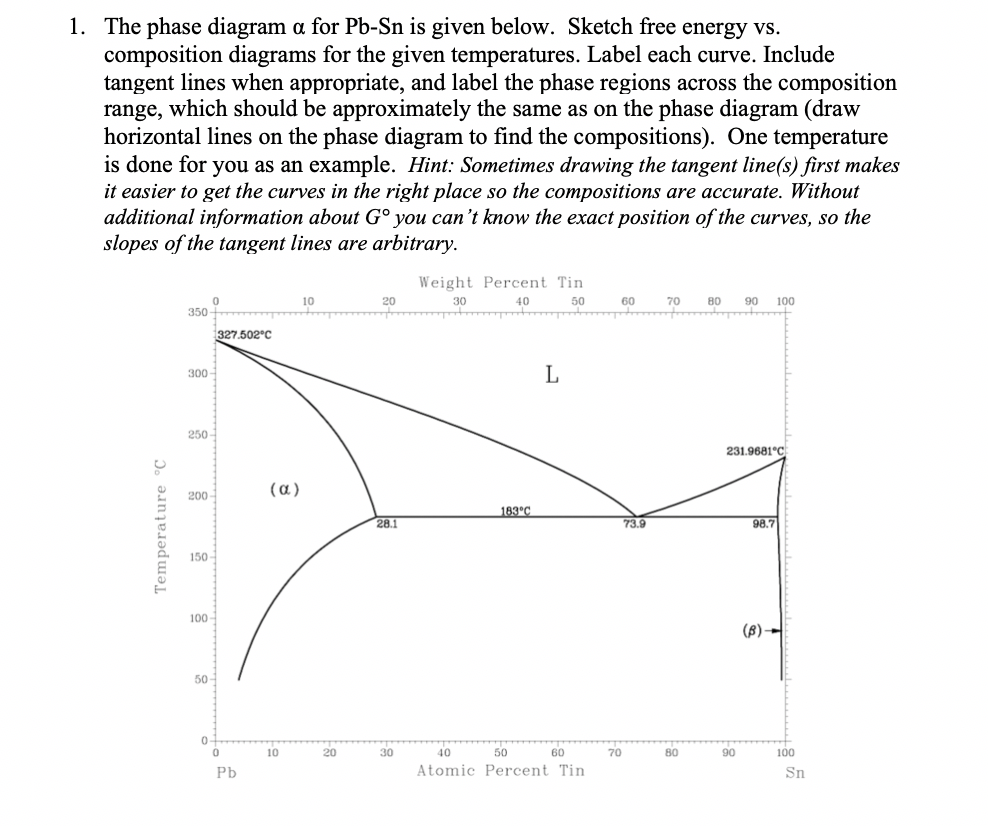
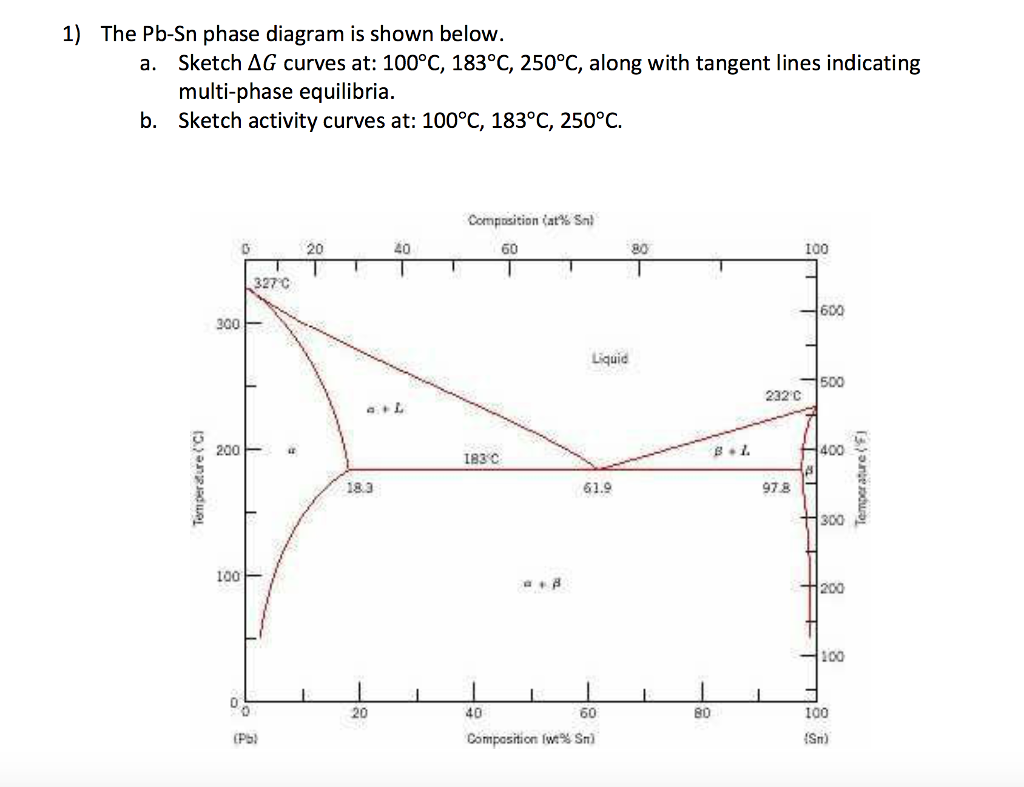
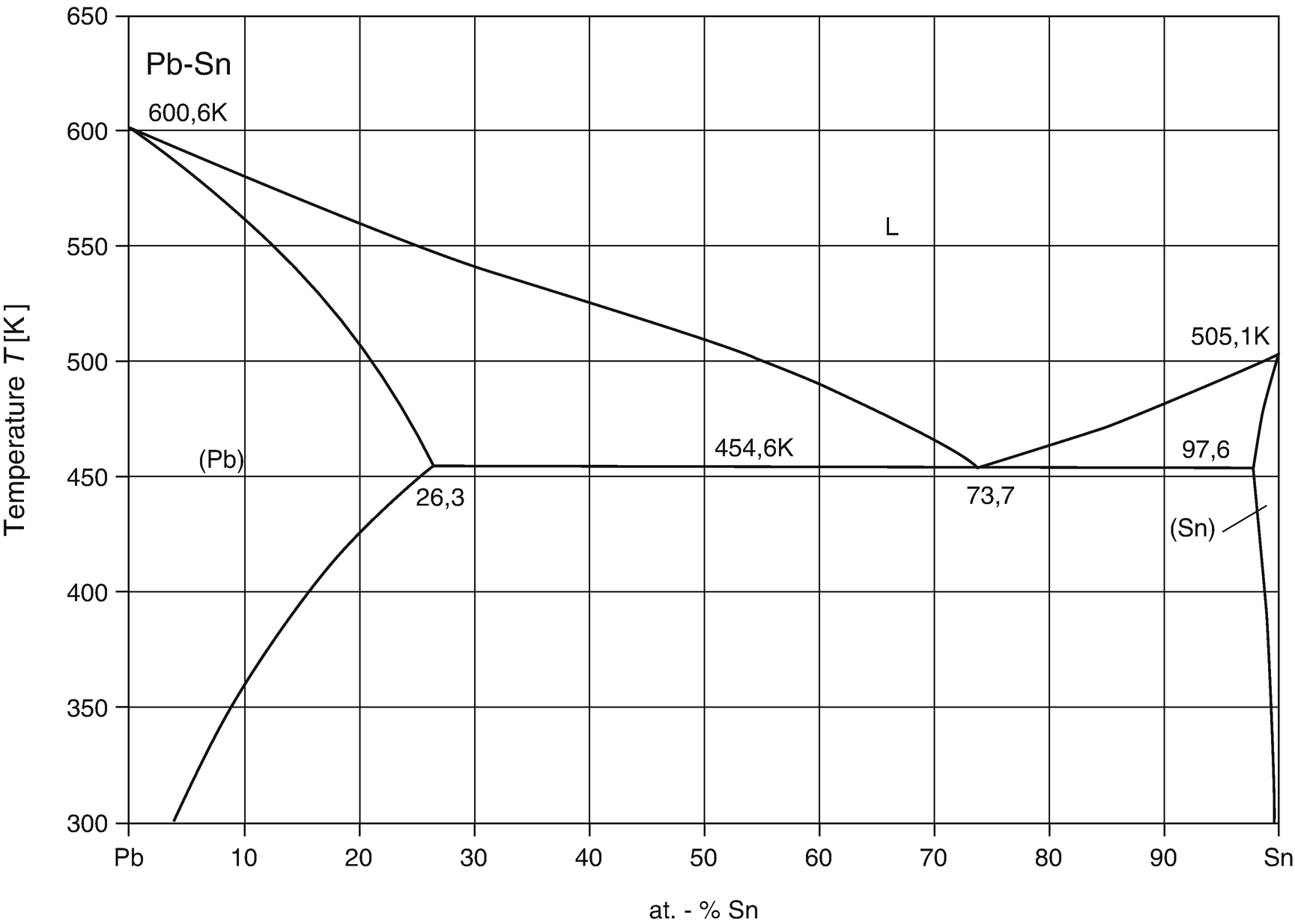
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash Figure 1 shows the Pb-Sn phase diagram and the composition for present experiments, in which it is very clear that the eutectic temperature is 183 • C, and the liquid-solid transition temperature... Watch this video lecture (Lecture 10) in Material Science at Mech Online Lectures to know about Lead Tin ( Pb-Sn ) Phase Diagram. Simple explanation of Eutec... The Attempt at a Solution. It is probably an easy question, but I thought it is a good idea to consult first. a) At 183 C, the first liquid phase forms. b) We can draw a tie line and the point intersects with the liquidus line, gives us the composition of liquid. It is 61.9 wt % Sn. c) It is around 250 C. Because phase diagram is on liquidus line.
In the Pb-Sn phase diagram Pb is component A and Sn is component B. microstructural component A component of the microstructure that has an identifiable characteristic morphology that developed due to its composition and thermal treatment microstructure Structure of grains and phases under magnification two-phase region
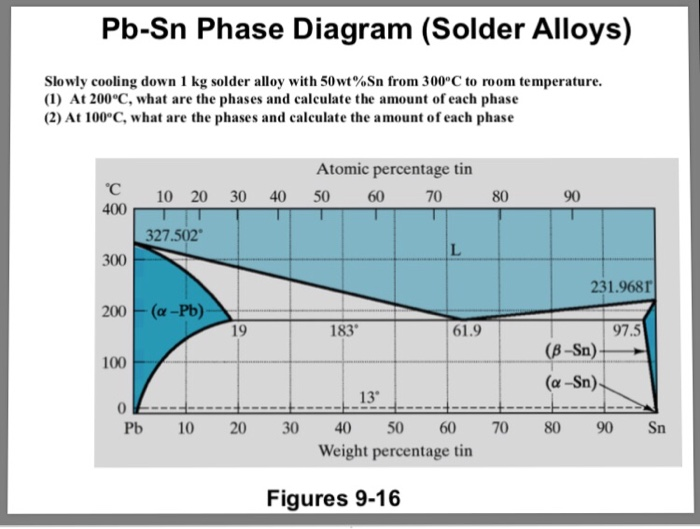
Example using the Pb-Sn Phase Diagram. Consider a 40 wt% Sn-60 wt% Pb alloy on the lead-tin phase diagram. Part 1: At 183.1 degrees C, just above the eutectic line, a) what phase(s) is (are) present? b) what is (are) the compositions of the phase(s)? c) what is the relative amount of each phase present, in mass fraction?
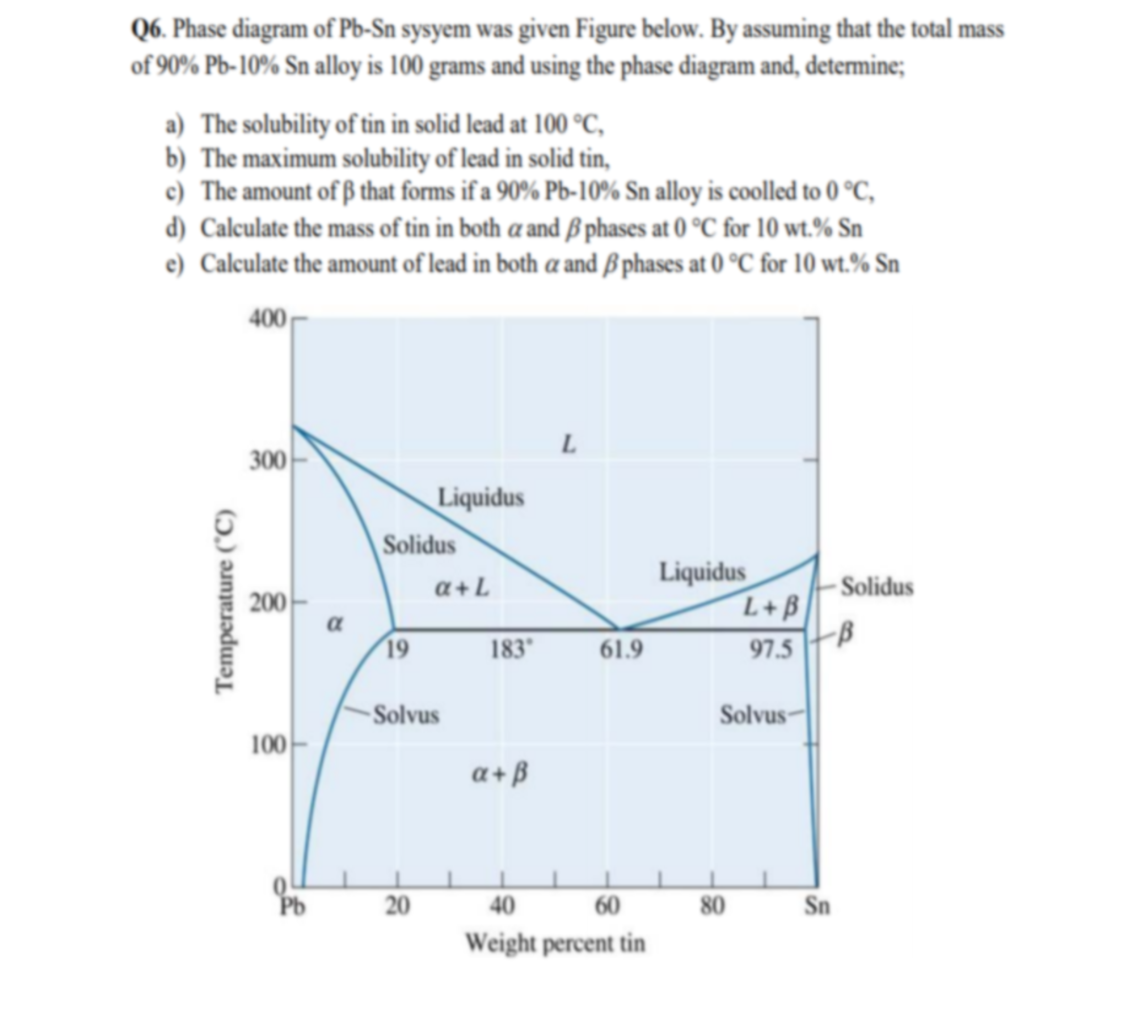
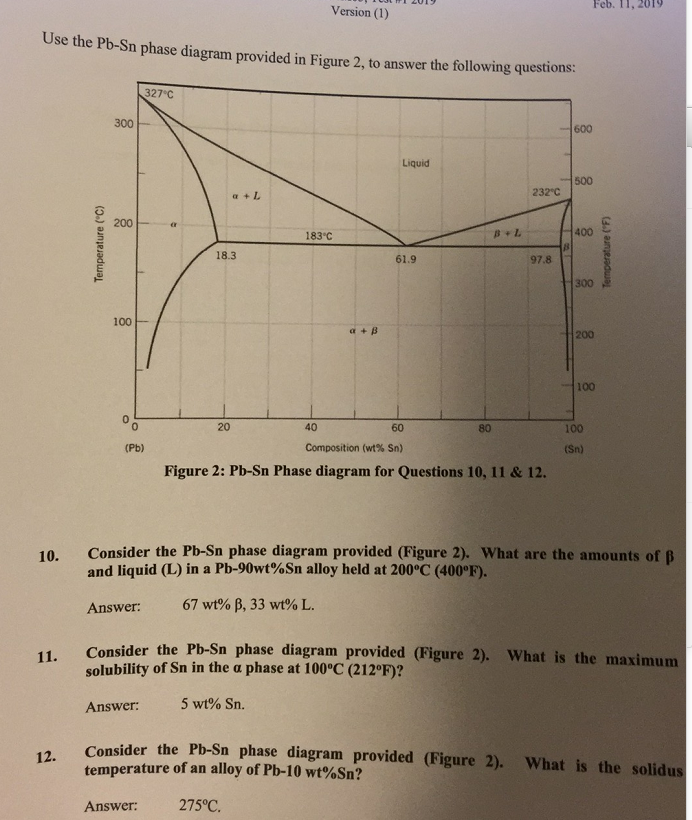
Question: 1) Using the Sn-Pb equilibrium phase diagram shown in the figure below and applying the lever rule, answer the following question. --- a) What is the eutectic composition? --- b) What are the compositions of the solid phases in equilibrium at the eutectic.
Phase Struktur-bericht Symbol Common Names Prototype Spacegroup Model * Liquid: n/a: L, L 1, L 2: n/a: n/a (Bi,Cu,Pb) 1 : Fcc: A1 (Cu), (Pb) Cu: Fm-3m (Cu,Pb,Sn) 1 (Va) 1: Bcc: A2 (beta Cu), beta: W: Im-3m (Cu,Sn) 1 (Va) 3: Bct: A5 (Sn), (beta Sn) beta Sn: I4 1 /amd (Cu,Pb,Sn) 1 : Cu 3 Sn.h: D0 3: gamma: BiF 3: Fm-3m (Cu,Sn) 0.75 (Cu,Sn) 0.25 ...
The study of ternary Ag-Pb-Sn phase diagram is important e.g. for the soldering industry. Pb-Sn alloys have been used as solders for a long time [1,2]. Even though the eutectic Pb-Sn has been prohibited from use in electronic products since 2006 [3], Pb-Sn alloys with Pb content higher than 85% are still in use [4].
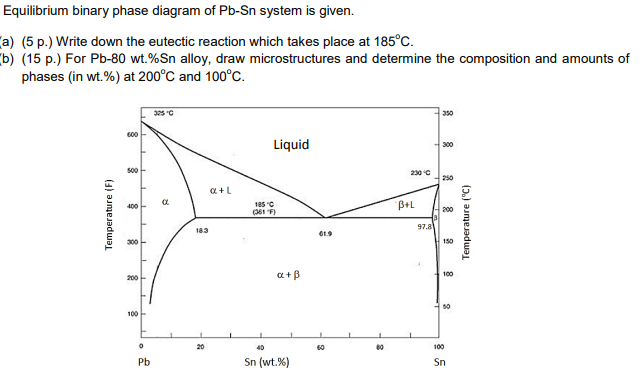
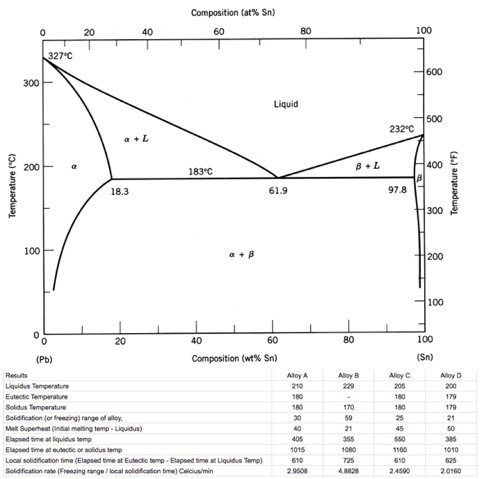
(1) The Pb-Sn phase diagram shown below is used as a basis for making different compositions of solders. 20 327.c 300 200 8.3 too 20 40 Composition Sr') 600 Liquid 232.c 97.8 300 200 too 183 oc 62.9 40 60 80 Sr..) (a) List all the components and phases in the phase diagram?
Pb Sn Phase Diagram Chapter 10 Phase Diagrams. Pb Sn Phase Diagram Morphology And Phase Formation During The Solidification Of Al Cu Si. Pb Sn Phase Diagram Solved 4 For The Pb Sn Phase Diagram Shown Below Answe. Pb Sn Phase Diagram Material Engineering Lecture Phase Diagrams Eutecticpart 2 Docsity.
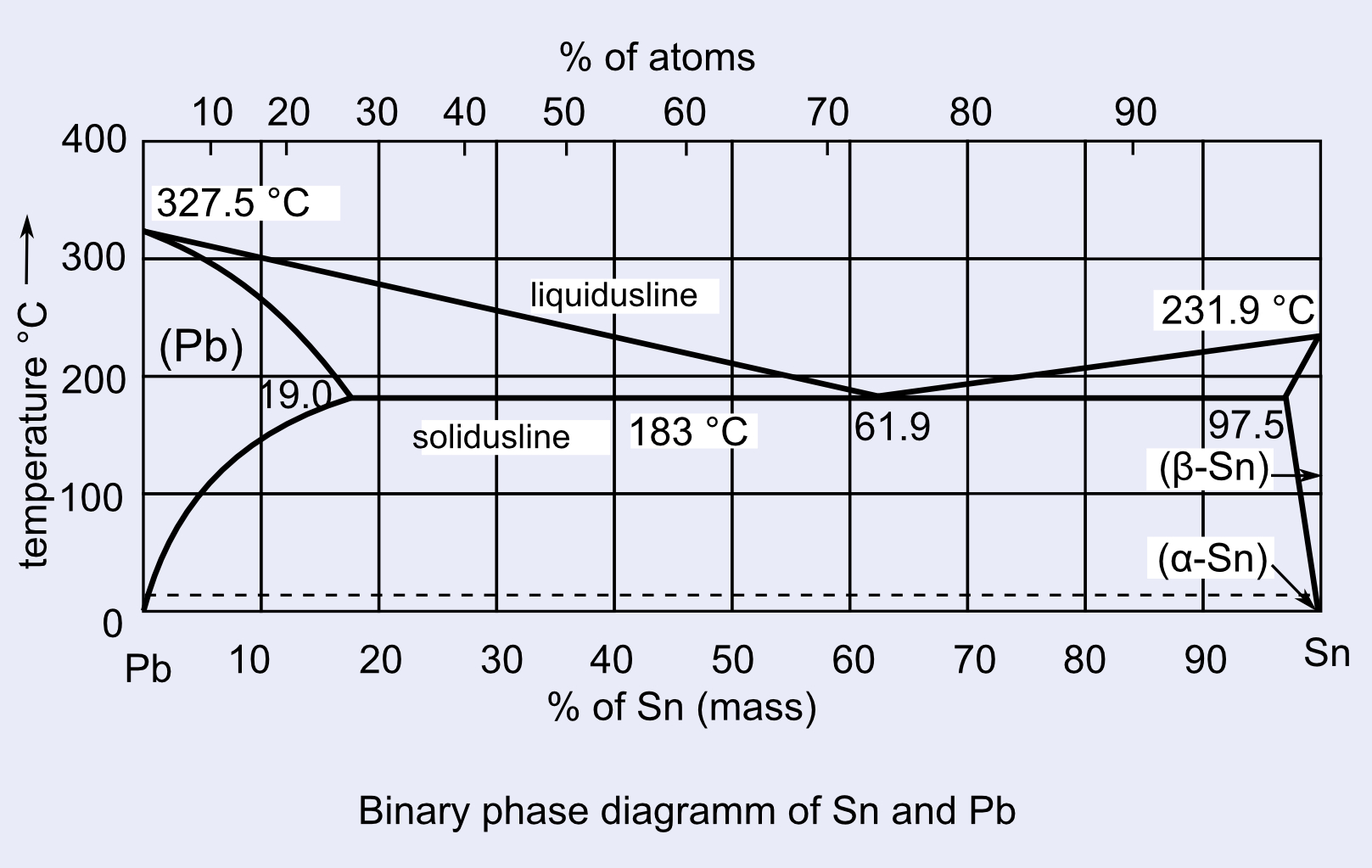
The Pb-Sn phase diagram calculated with the data from Ref. [ 38 ]. 3.3. The solution phases The bct (β-Sn, labeled Sn in consequent text) and diamond (α-Sn) phases were modeled as substitutional solid solutions. The LIQUID phase was also modeled using a substitutional model with one sublattice.
the Pb Sn-Te phase diagram is four-dimensional and can be more conveniently represented by four projec- tions: 7-2. -r,, p-T z, p-T-y, and p ; y. In each of these projections, two-phase equilibria...
Pb-Sn Phase Diagram Pb (lead) Sn (Tin) Temperature, °F 0 100 200 300 400 500 600 α α+L L β+L β α+β 362 °F 61.9% Sn Eutectic Composition and Temperature Time α 5 Fe-C Phase Diagram 200 600 1000 1400 1800
We will use the Pb-Sn phase diagram as an example. Pb-Sn alloys are used as common solders. A eutectic reaction is one in which the liquid phase solidifies to produce two solid phases. eg in the Pb-Sn eutectic system, Liquid reacts to form α + , ie L α + . Eutectic systems are relatively simple and common.
Pb-Sn Phase Diagram Liquidus Solidus Solidus Solidus Solvus Solvus 28. 28 Solidification of Eutectic Mixtures • Mixtures of some metals, such as copper & nickel, are completely soluble in both liquid and solid states for all concentrations of both metals. Copper & nickel have the same crystal structure (FCC) and have nearly the same atomic radii.
Pb-Sn phase diagram β phase: solid solution of Pb in tetragonal Sn α phase: solid solution of Sn in fcc Pb Liquid Pb (Fcc) Sn (Tetra) 0 50 100 150 200 250 300 350 0 10 20 30 40 50 60 70 80 90 100 T emperature Wt% The Pb-Sn system is characteristic of a valley in the middle. Such system is known as the Eutectic system. The central point is the Eutectic
(b) That portion of the Pb-Sn phase diagram (Figure 9.8) that pertains to this problem is shown below; the point labeled “B” represents the 75 wt% Sn-25 wt% Pb composition at 175°C. As may be noted, point B lies within the α + β phase field. A tie line has been constructed at 175°C; its intersection





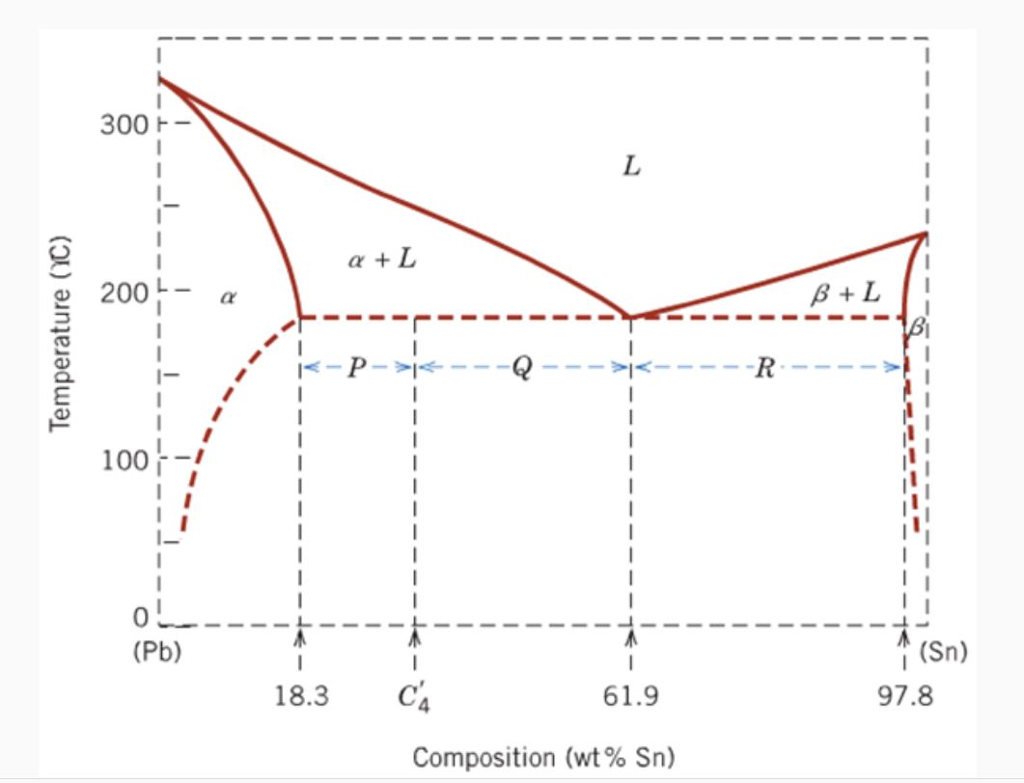

















0 Response to "45 pb sn phase diagram"
Post a Comment