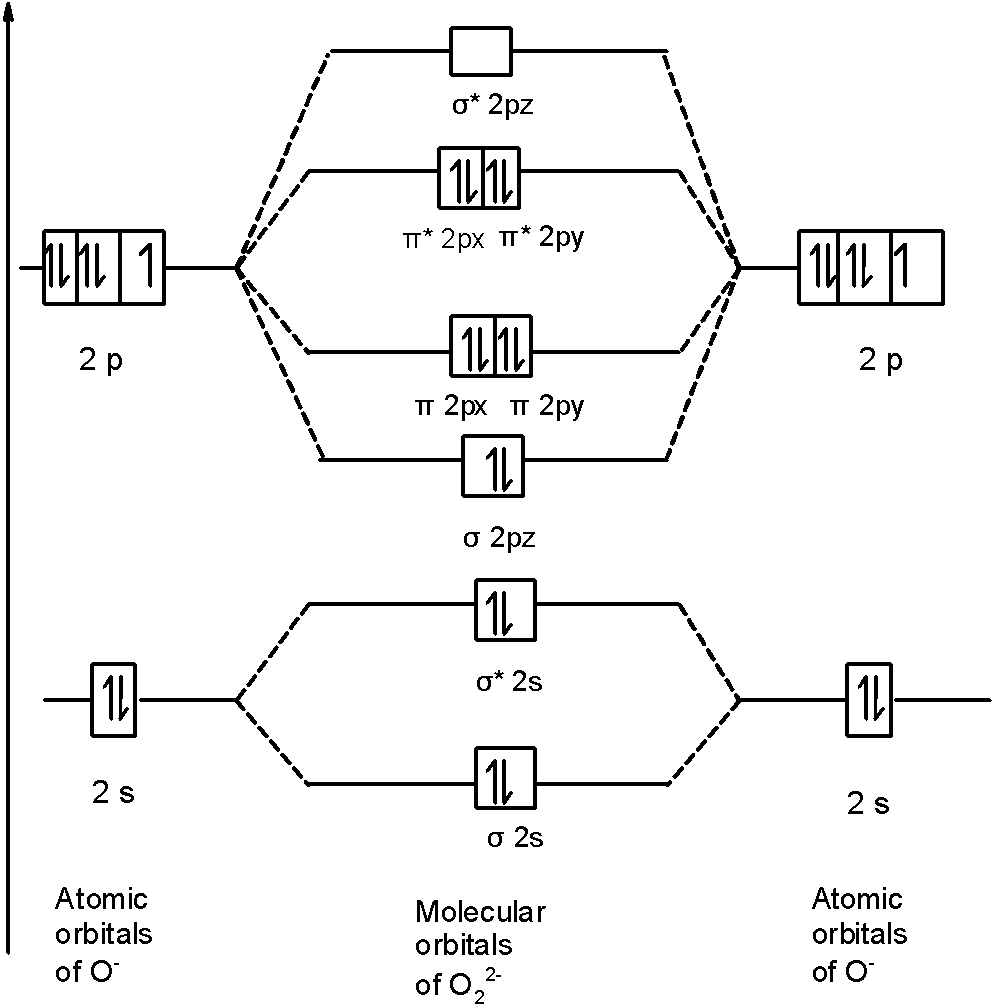
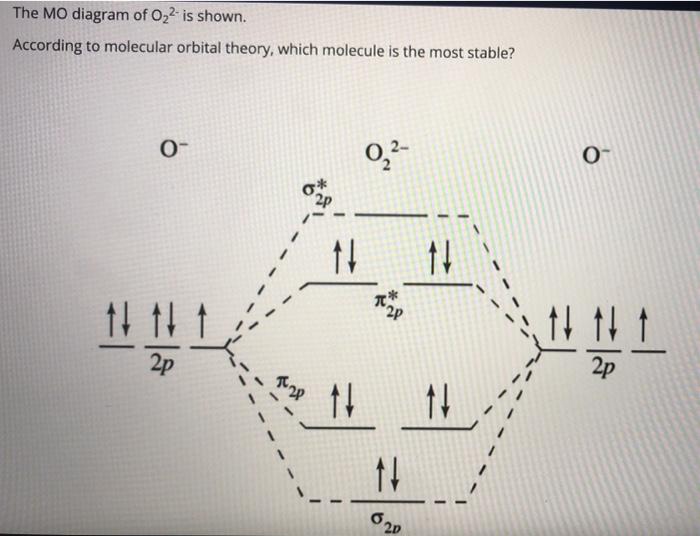
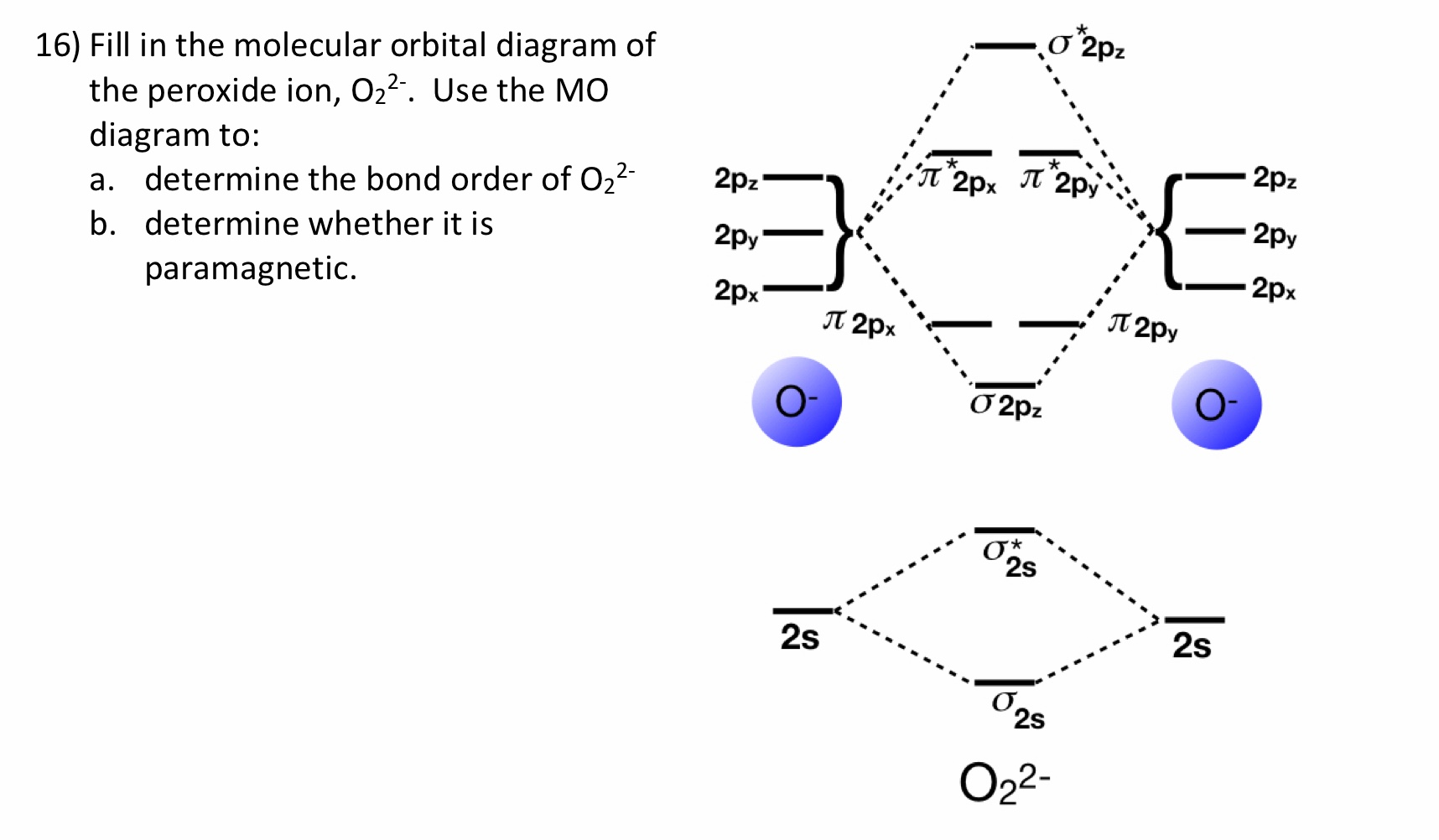
43 o2 2- mo diagram
MO Diagram for O2(2-) - YouTube It is sigma2s(2)sigma2s*(2)sigma2p(2)pi2p(4)pi2p*(4)Bond order 1. It is stable. In fact, it's the perioxide ion.Check me out: Based on MO theory compare the relative stabilities of O2 ... Click here👆to get an answer to your question ️ Based on MO theory compare the relative stabilities of O2 & O2^2 - and indicate their magnetic properties.
en.wikipedia.org › wiki › OxygenOxygen - Wikipedia 2 partial pressure in the breathing gas is, in general, about 30 kPa (1.4 times normal), and the resulting O 2 partial pressure in the astronaut's arterial blood is only marginally more than normal sea-level O 2 partial pressure. Oxygen toxicity to the lungs and central nervous system can also occur in deep scuba diving and surface supplied diving.

O2 2- mo diagram
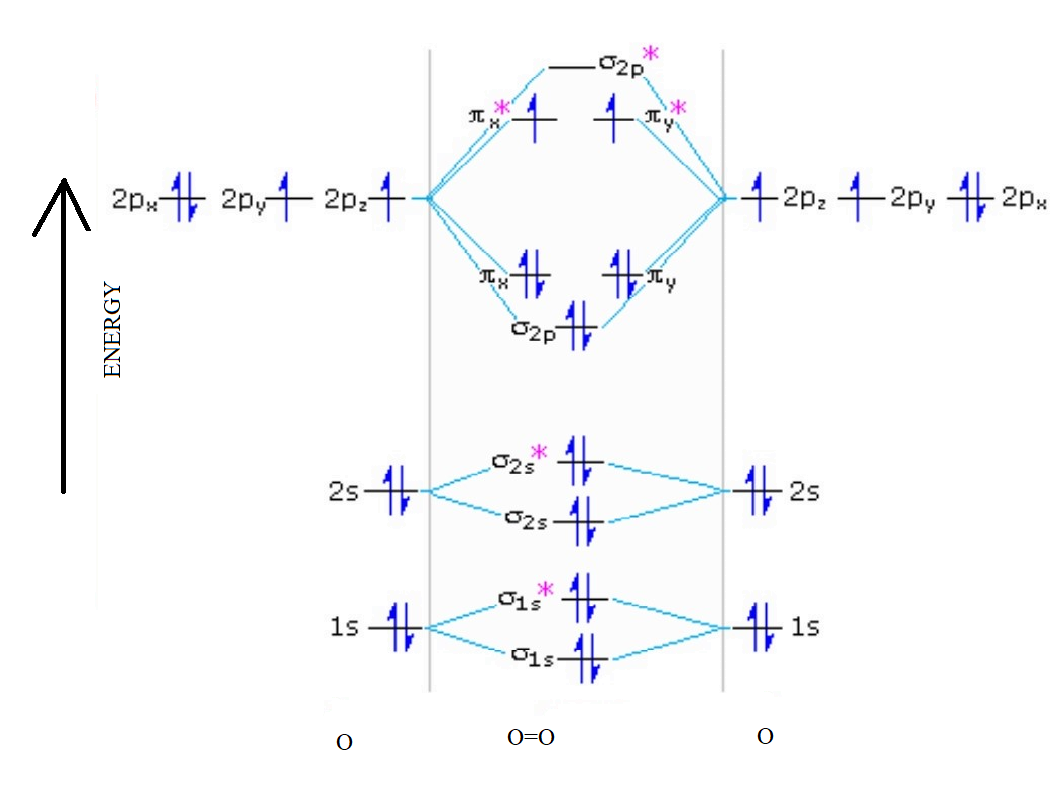
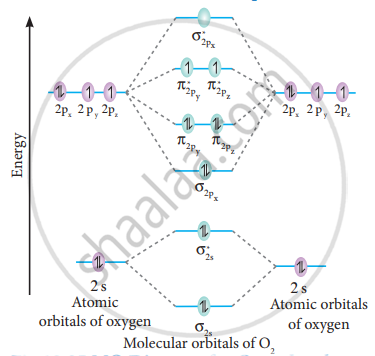
Explain the formation of O2 molecule using molecular class ... $1{s^2}2{s^2}2{p^4}$ One atom of oxygen has 8 electrons. Thus, two atoms will possess 16 electrons i.e. Oxygen molecules will have 16 electrons. The molecular orbital diagram of an Oxygen molecule is as - N2+ Mo Diagram 2- = Molecular orbital for N2, N2+, O2, H2 and He2 by Thomas Wells - December 5, Brian Verfuerth 0. Ozone Lewis diagrams and by avatar Claire Bridget . The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves. Draw molecular orbital diagram of O2 or N2 with magnetic ... Draw molecular orbital diagram of O2 or N2 with magnetic behavior and bond order. Medium Open in App Solution Verified by Toppr As it can be seen from the MOT of O2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [Nb −Na ]/2=[10−6]/2=2.
O2 2- mo diagram. PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine CN- lewis structure, molecular orbital diagram, and, bond ... Also, using the Molecular orbital diagram of CN-we can also find its bond order which helps us to predict its bond length and stability as well. Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2. electronic configuration - Molecular orbital (MO) diagram ... Show activity on this post. I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. For N X 2 the orbitals in increasing energy are: σ 1 s < σ 1 s ∗ < σ 2 s < σ 2 s ∗ < π 2 p x, π 2 p y < σ 2 p z < π 2 p x ∗, π 2 p y ∗ < σ 2 p z ∗ ... Draw a molecular orbital diagram of N2 or O2 with magnetic ... Molecular orbital theory is a method for describing the electronic structure of the molecule. Now, let us draw the molecular orbital diagram of ${N_2}$ . Now, first let us understand what magnetic behavior and bond order means.
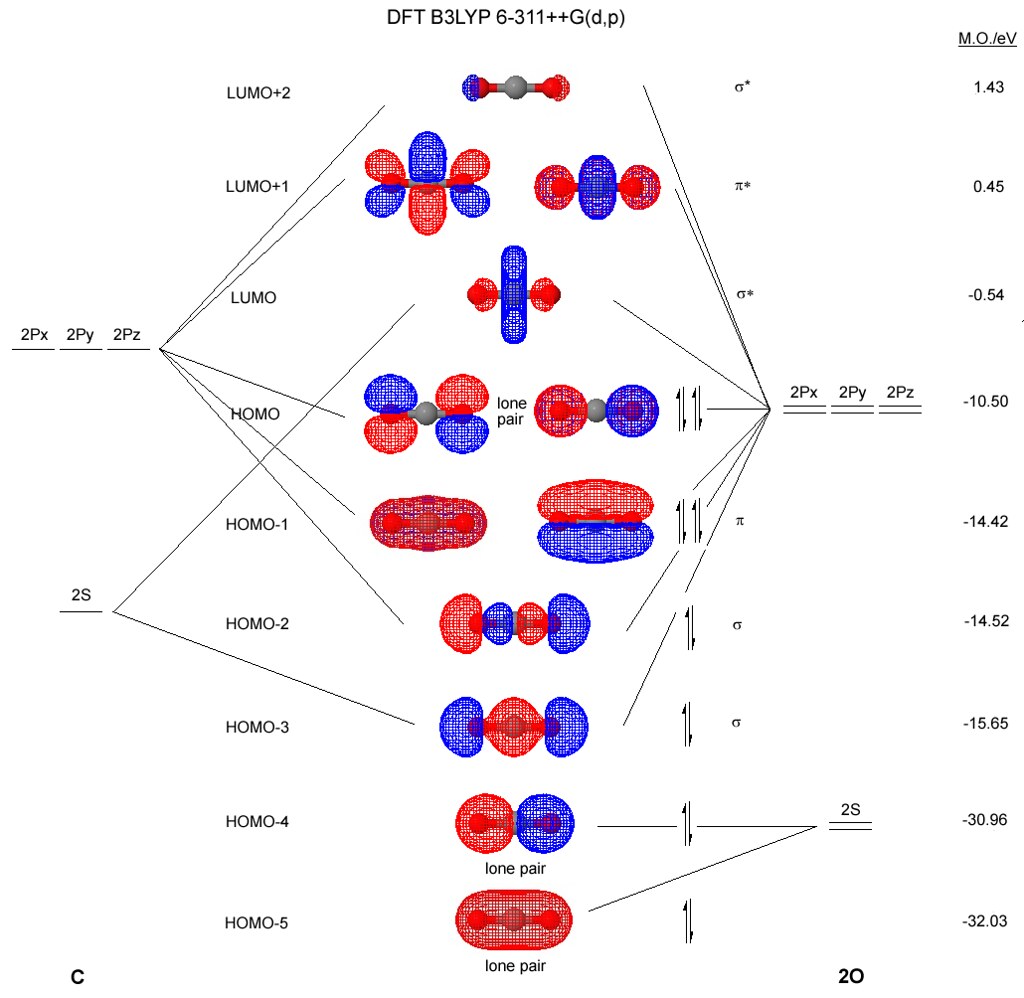
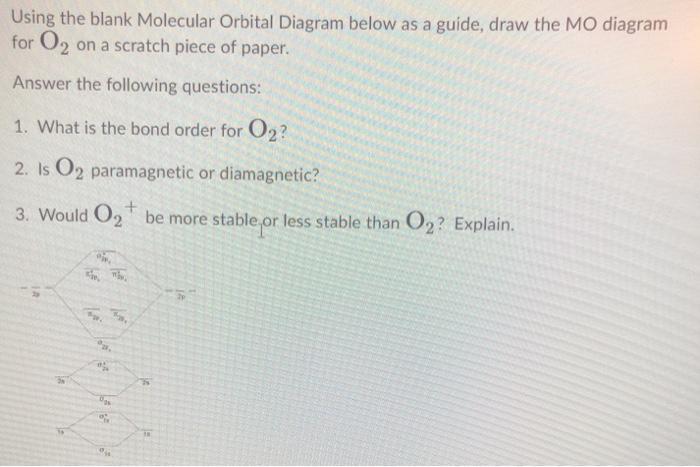
Explain the formation of O2 molecule using molecular ... The molecular orbital energy level diagram of oxygen molecule is given as follows : Bond order 2 N b − N a = 2 8 − 4 = 2 Thus, oxygen molecule has two bonds. i.e., one is bond and one p bond. materialsproject.org › materials › mp-352mp-352: HfO2 (monoclinic, P2_1/c, 14) - Materials Project Hf4+ is bonded to seven O2- atoms to form a mixture of distorted corner and edge-sharing HfO7 pentagonal bipyramids. There are a spread of Hf–O bond distances ranging from 2.05–2.26 Å. There are two inequivalent O2- sites. In the first O2- site, O2- is bonded in a distorted trigonal non-coplanar geometry to three equivalent Hf4+ atoms. Molecular Orbital (MO) Diagram of O2 - YouTube Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*... O2 2- (Peroxide) Ion Lewis Structure Peroxide (O2 2-) anion has -2 charge. In O2 2- lewis structure, each oxygen atom has -1 charge and three lone pairs. Both oxygen atoms are joint through a single bond. In this tutorial, we are going to draw the lewis structure of peroxide ion step by step.
› PneumothoraxPneumothorax - Physiopedia Prevalence of a pneumothorax in a newborn is a potentially serious problem and it occurs in about 1-2% of all births. [2] The overall person consulting rate for pneumothorax (primary and secondary combined) in the GPRD was 24.0/100 000 each year for men and 9.8/100 000 each year for women. PDF MO Diagrams for Linear and Bent Molecules Relative AO Energies in MO Diagrams Use AO energies to draw MO diagram to scale (more or less). H He Li Be B C N O F Ne B C N O F Ne Na Mg Al Si P S Cl Ar Al Si P S Cl Ar 1s 2s 2p 3s 3p -19.4 eV -15.8 eV -32.4 eV -10.7 eV Using the MO diagram of "NO", calculate the bond order ... The MO diagram for "NO" is as follows (Miessler et al., Answer Key): (The original was this; I added the orbital depictions and symmetry labels. For further discussion on the orbital energy ordering being "N"_2-like, see here and comments.) Quick overview of what the labels correspond to what MOs: 1a_1 is the sigma_(2s) bonding MO. 2a_1 is the sigma_(2s)^"*" antibonding MO. 1b_1 is the pi_(2p ... Solved 2.10 (a) Construct an MO diagram for the formation ... 2.10 (a) Construct an MO diagram for the formation of O2; use only the valence orbitals of the oxygen atoms. (b) Use the diagram to rationalize the following trend in 0-0 bond distances: O2, 121 pm; [O2]*, 112 pm; [02], 134 pm; [O2], 149 pm. (c) Which of these species are paramagnetic? ah of the
MO DIAGRAM O2+ , O2 2+ ,O2- ,O2 2- (preparation of gate ... Follow me on instagram- me on facebook page- ...
N2+ Mo Diagram - schematron.org There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc) . One is for the elements up to Nitrogen. The other is for AFTER nitrogen. The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves. Bond order for N2 is 3; bond order for N2- is and bond ...
The increasing order of the bond order of O2, O2-, O2+ and ... Bond order (B.O) 1/2 × [Number of an electron in antibonding molecular orbitals] - [Number of electrons in bonding molecular orbitals] The higher the order of the bond the greater the pull between the two atoms and the shorter the length of the bond. (1) B.O for O 2 = 1/2 × [10 - 6] B.O for O 2 = 2 (2) B.O for O 2 - = 1/2 × [10 - 7]
PDF MO-Schema O2 - uni-potsdam.de MO-Schema O 2-(Superoxid)Energie 2p 2s 1s 1s * 1s b 2p 1s 2s b 2s * 2s b p x b p z *p x. Title: PowerPoint-Präsentation Author: A.Kelling Created Date: 6/30/2011 9:20:45 AM
mp-12957: O2 (monoclinic, C2/m, 12) - Materials Project O2 is alpha oxygen-like structured and crystallizes in the monoclinic C2/m space group. The structure is zero-dimensional and consists of eight hydrogen peroxide molecules. O is bonded in a single-bond geometry to one O atom. The O–O bond length is 1.23 Å.
By writing molecular orbital configuration for NO,CO,O2 ... 18.3.2018 · Also see here... Bond order for "NO"^+ Order by bond length: "NO", "NO"^(+), "NO"^(-) Is "CO" a Lewis acid? "O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The bond order is …
What's the MOT diagram of O2 +2 ion? - Quora Answer (1 of 3): In O2 2+, there is 14 electrons. So, it's MOT is comparable to N[code ]2[/code] & the MOT diagram will look like this :
He2 2+ Molecular Orbital Diagram - schematron.org A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ...
O2 -2 MO Diagram - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
PDF Energetic and chemical reactivity of atomic and molecular ... The molecular orbital diagram can be constructed from the molecular orbital theory (Figure 2). In molecular oxygen, there are 16 electrons which can be placed into the molecular orbitals to give the electronic configuration: (σ1s) 2(σ* 1s) 2(σ 2s) 2(σ* 2s) 2(σ 2p) 2 (σ* 2p) 2(π 2p) 4(π* 2p) 2. This electronic configuration shows that ...
MO Diagrams - University of Sydney Molecular Orbital Diagram Maker. These quizzes enable you to build your own molecular orbital diagram from components. A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons.
mp-1094034: Ti3C2 (hexagonal, P6_3/mmc, 194) Ti3C2 crystallizes in the hexagonal P6_3/mmc space group. The structure is two-dimensional and consists of two Ti3C2 sheets oriented in the (0, 0, 1) direction. there are two inequivalent Ti+2.67+ sites. In the first Ti+2.67+ site, Ti+2.67+ is bonded in a distorted T-shaped geometry to three equivalent C4- atoms. All Ti–C bond lengths are 2.07 Å.
chemed.chem.purdue.edu › genchem › topicreviewMolecular Orbital Theory - Purdue University The molecular orbital diagram for an O 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals. Molecular Orbitals of the Second Energy Level
SO2 Lewis Structure, Hybridization, Molecular Geometry ... Feb 23, 2022 · SO2 Molecular Orbital Diagram. The molecular orbital diagram of SO2 is attached below: A molecular orbital diagram gives us an idea about how the atomic orbitals of two different atoms can fuse and give rise to a new orbital. This further helps us to find out the bond order, bond length, and bond strength of any compound. In this MO we can see ...
Draw the molecular orbital energy diagram for oxygen ... Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown:(i) Electronic configuration:(ii) Bond order: Here Nb = 8; Na = 4The two oxygen atoms in a molecule of oxygen are united through two covalent bonds ...
Molecular Orbital (MO) Diagram for O2(2+) - YouTube Remember: When two oxygen atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals. They are flipped compare...
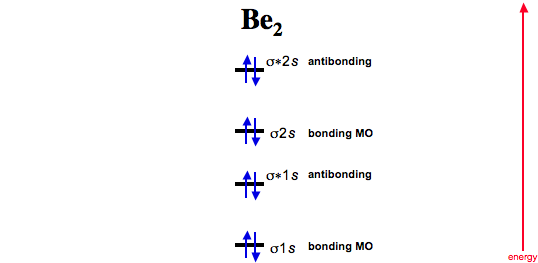
Solved Draw the MO diagram for Li2, Be2, B2, C2, N2 ... Draw the MO diagram for Li 2, Be 2 , B 2, C 2, N 2, N 2 +, O 2, O 2+2 , O 2-2, Ne 2, NO, NO -, CO, and CN - (list if it is paramagnetic or diamagnetic if possible) Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
Draw molecular orbital diagram of O2 or N2 with magnetic ... Draw molecular orbital diagram of O2 or N2 with magnetic behavior and bond order. Medium Open in App Solution Verified by Toppr As it can be seen from the MOT of O2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [Nb −Na ]/2=[10−6]/2=2.
N2+ Mo Diagram 2- = Molecular orbital for N2, N2+, O2, H2 and He2 by Thomas Wells - December 5, Brian Verfuerth 0. Ozone Lewis diagrams and by avatar Claire Bridget . The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves.
Explain the formation of O2 molecule using molecular class ... $1{s^2}2{s^2}2{p^4}$ One atom of oxygen has 8 electrons. Thus, two atoms will possess 16 electrons i.e. Oxygen molecules will have 16 electrons. The molecular orbital diagram of an Oxygen molecule is as -























0 Response to "43 o2 2- mo diagram"
Post a Comment