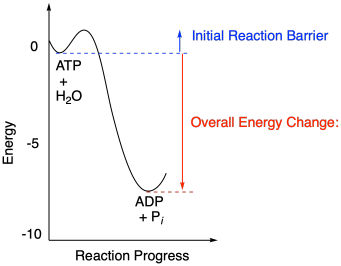
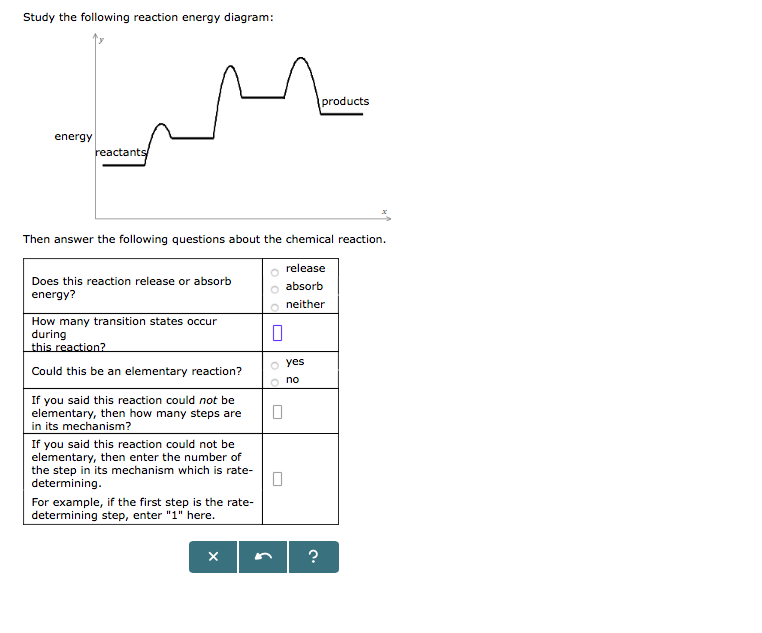
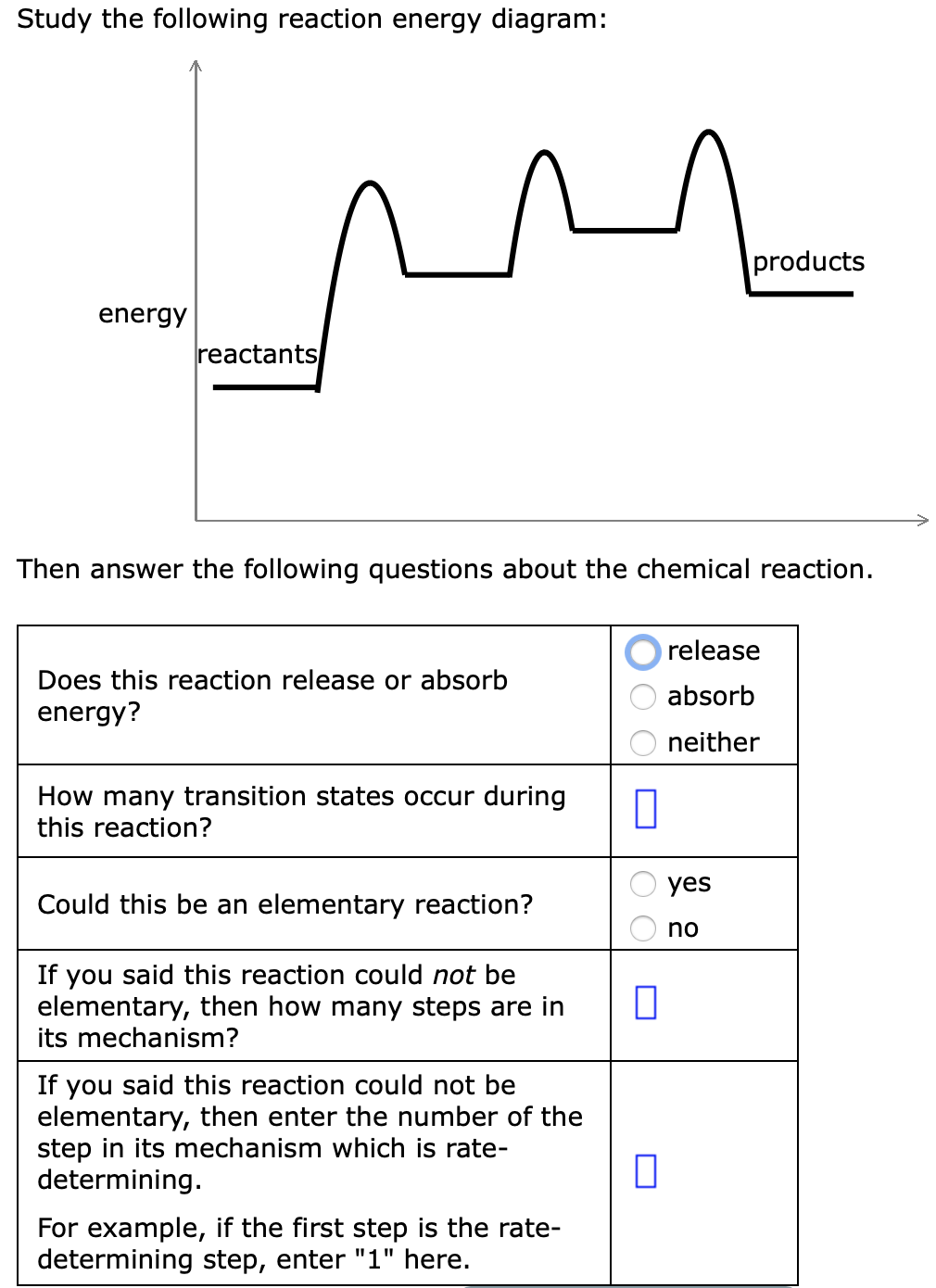
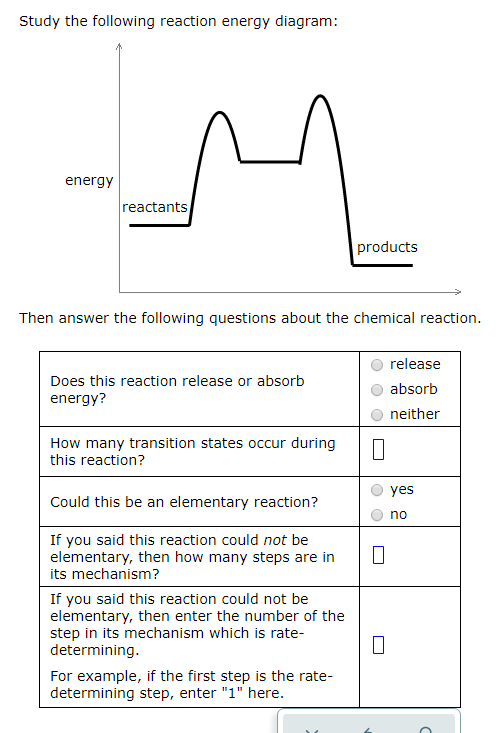
45 study the following reaction energy diagram:
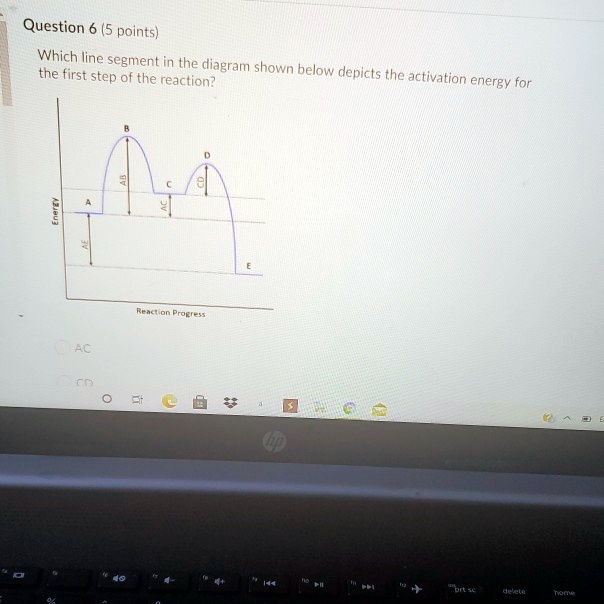
1i. Draw an energy vs reaction coordinate diagram to illustrate a reaction in which the energy of the products is greater than the energy of the reactants. Label all quantities as per Fig. 1. See diagram (3) in sample exercise 14.10 on pg 595 of Brown and LeMay, 11th ed. Which of the following statements is not true? A) Two reactions can have identical values for DH° but very different Ea values. B) The larger the activation energy, the slower the reaction. C) DH° determines the height of the energy barrier. D) The lower the activation energy, the faster the reaction.
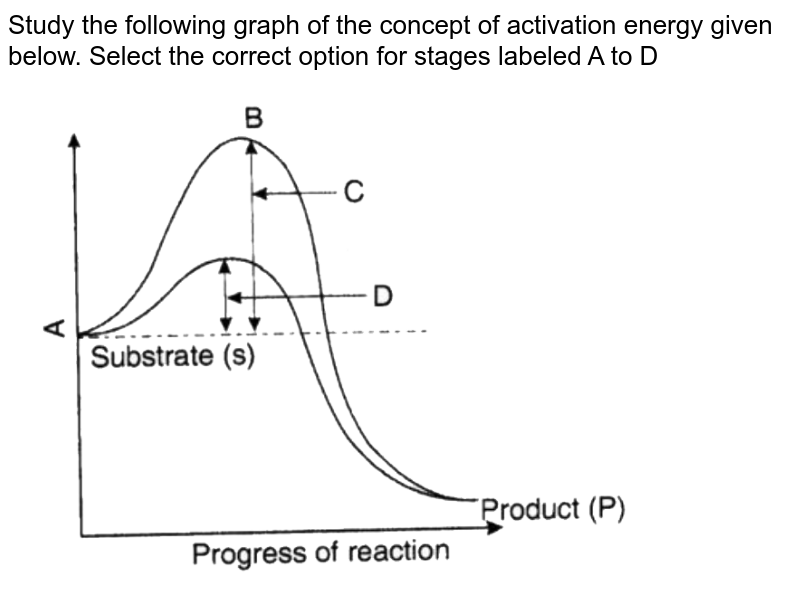
It decreases the kinetic energy of reactants, enabling them to undergo chemical change more easily. Correct Answer: By lowering the activation energy, an enzyme increases the rate of the reaction. Review B. 4. Consider the following energy diagram for a chemical reaction.
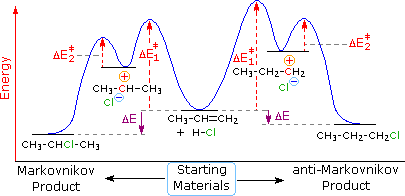
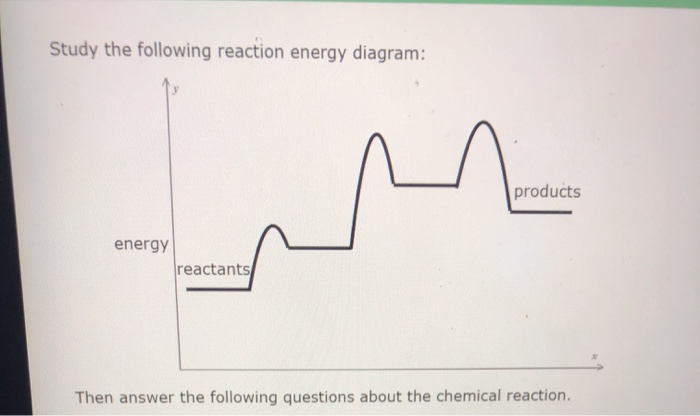
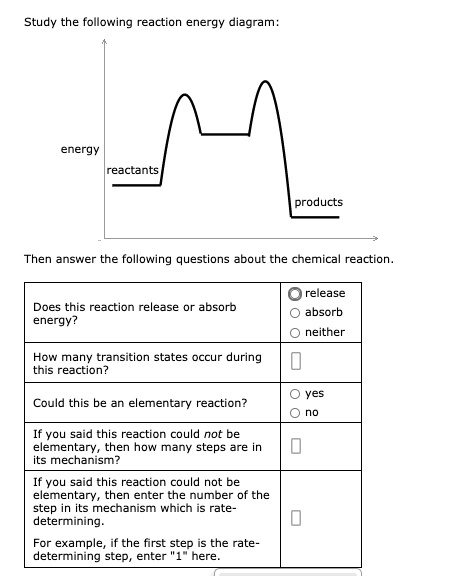
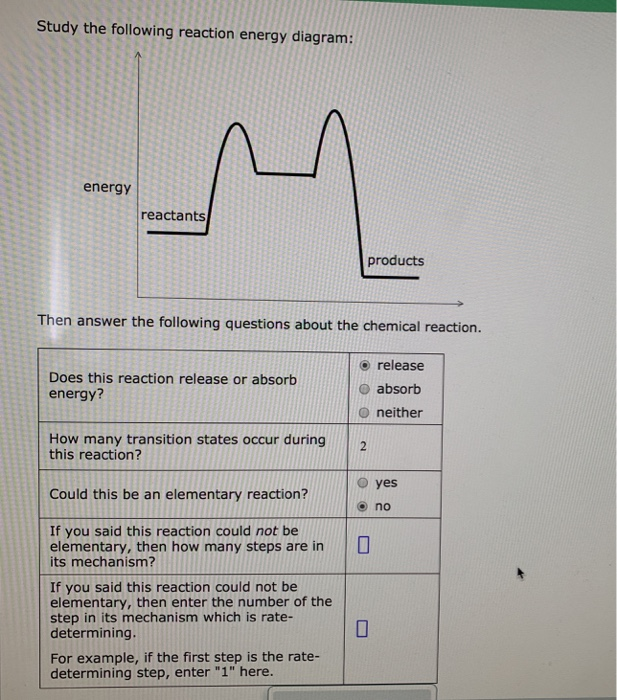
Study the following reaction energy diagram:
All reactions require energy, from the simplest - such as boiling water to turn liquid water into gaseous water - to the complex, such as electrochemical reactions. We can see what is happening to... Use the following information to answer the next question. Titanium is widely used in the aerospace and sporting goods industries because it is strong, lightweight, and resistant to corrosion. When exposed to oxygen, titanium forms an oxide coating that protects the metal from corrosion. This reaction is represented by the following equation. The energy change in the water bath will be the _____ magnitude in energy as the chemical reaction or phase change, just with the _____. If the water bath gains energy, its temperature goes _____, meaning the energy of the chemical reaction or phase change went down (it lost energy) and vice versa.
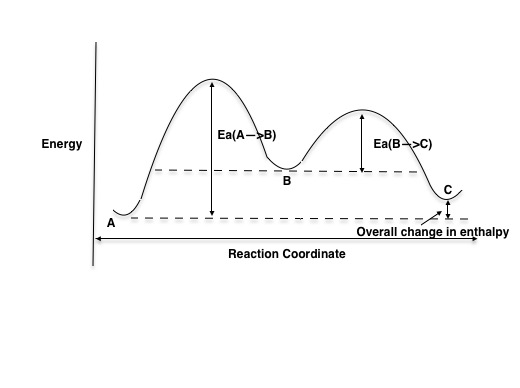
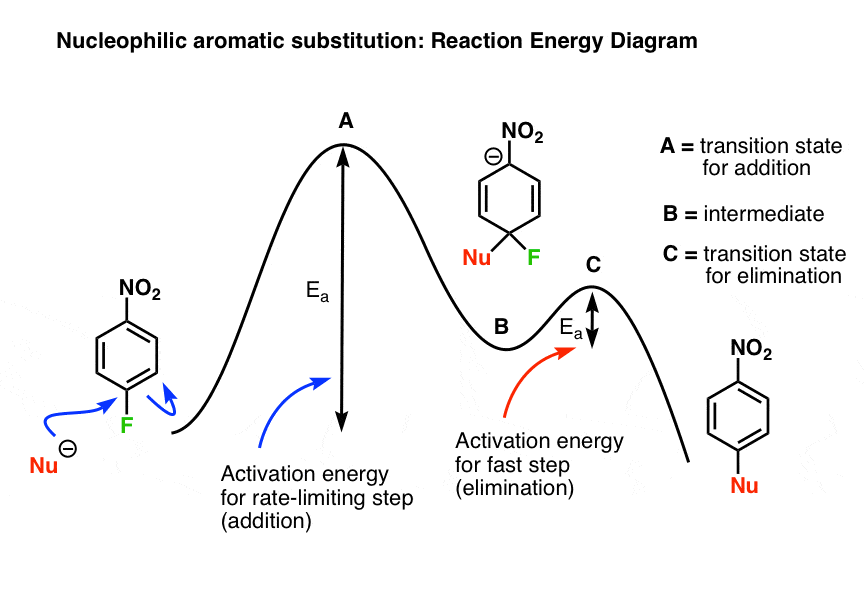
Study the following reaction energy diagram:. b) Does the reaction proceed via S N 1 or S N 2 mechanism? c) Draw the products and complete mechanism of the reaction and identify the rate determining step. d) Is there an intermediate in this reaction? If yes, show its structure and position on the energy diagram. e) How many kJ is the activation energy of the rate determining step? The energy distribution profile (Curve C) for the Y2 molecules is shown in the graph above for the reaction X+Y2→XY2 when it is done under certain experimental conditions. Line A represents the most probable energy of the Y2 molecules, and Line B represents the activation energy. Match each reaction with its correct energy diagram. он он H,SO, H2SO, HCI OH B) C) DIAGRAM 1 DIAGRAM 2 DIAGRAM 3 Answer the following questions regarding the kinetics of chemical reactions. (a) The diagram below at right shows the energy pathway for the reaction O3 + NO NO2 + O2. Clearly label the following directly on the diagram. (i) The activation energy (Ea) for the forward reaction (ii) The enthalpy change ( H) for the reaction
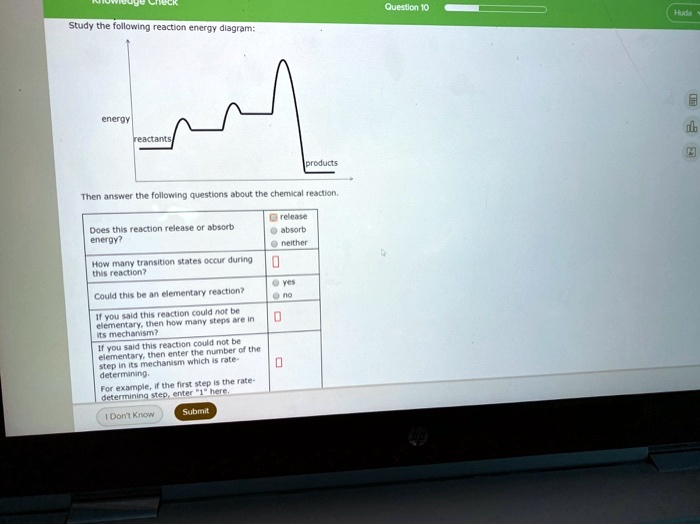
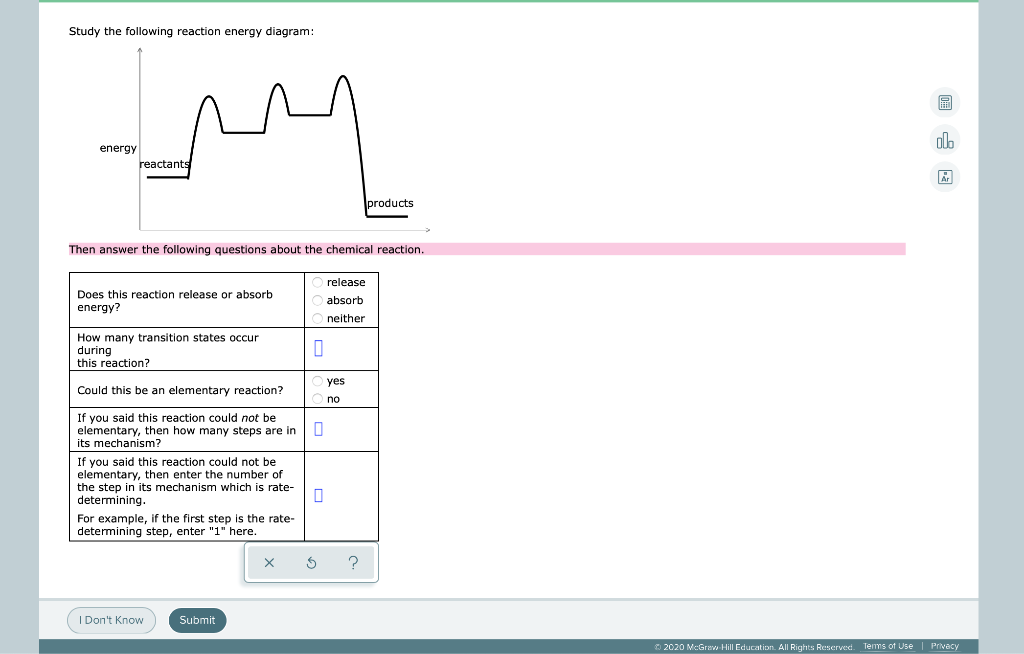
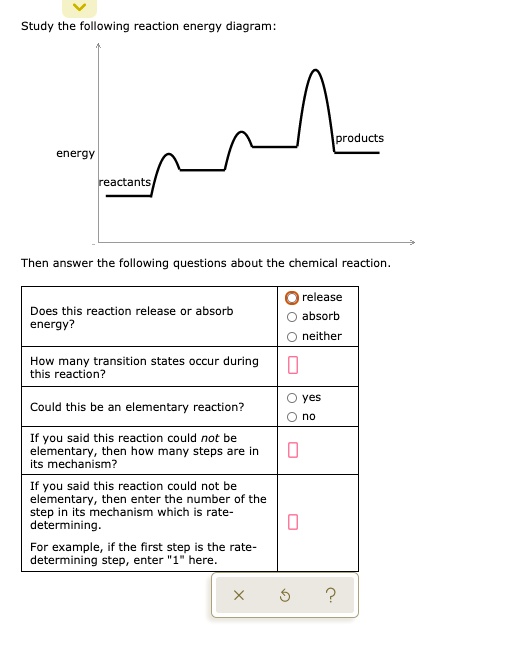
In drawing an enthalpy diagram we typically start out with the simplest part first, the change in energy. Let's say that we're looking at the chemical reaction of methane and oxygen burning into... Study the following reaction energy diagram: products energy reactants Then answer the following questions about the chemical reaction. release Does this reaction release or absorb energy? o absorb o neither How many transition states occur during this reaction? Could this be an elementary reaction? On the diagram below, draw a potential energy diagram for this reaction. 18. Base your answer(s) to the following question(s) on the reaction represented by the balanced equation below. 2H2(g)+O2(g) !2H2O(')+571:6kJ On the axes below, draw a potential energy diagram for the reaction represented by this equation. 19. Given the reaction at ... Which of the following statements correctly describe the key aspects of drawing a reaction energy diagram. For an exothermic reaction the energy of the products is less than the energy of the reactants.
Explanation: The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants. Here the energy of the reactant is less than the product i.e ; it is an endothermic reaction. Hence, in this reaction the energy is absorbed. Her …. View the full answer. Transcribed image text: Study the following reaction energy diagram: Does this reaction release or absorb energy? release absorb neither yes no 0 How many transition states ... Use the energy diagram for the rearrangement reaction of methyl isonitrile to acetoni- trile to answer the following questions. 7. What kind of reaction is represented by this diagram, endothermic or exothermic? 8. What is the chemical structure identified at the top of the curve on the diagram? Cor«plc¥ 9. What does the symbol E represent? 10. 5. Question 5 - The Energy Diagram of SN2 reaction: Draw an energy diagram for the following S N 2 reaction. Label the axes, the Ea, the ΔH° and the transition state of the reaction.Assume the reaction is exothermic and ΔH° = -75 kJ/mol and Ea = 50 kJ/mol. Draw the structure of reactants and products on the diagram.You can put the reactants at any energy level and then draw the rest as ...
Step-by-step explanation. Question (3 ) solution ( a) Energy diagram for Exothermic with high Eact is as shown below .. Activation energy potential energy Energy energy Released. of Reactant energy of product Progress of Reaction - Energy Released in Exothermic Reaction. ( b) Energy diagram for Endothermic with low Eact is as shown below .
Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.
Study the following reaction energy diagram: energy reactants, products Then answer the following questions about the chemical reaction. release Does this reaction release or absorb O absorb energy? O neither How many transition states occur during this reaction? O yes Could this be an elementary reaction?
7. Use the following diagram to answer the questions below: B Reaction Progress a) Is the reaction exothermic or endothermic? Explain. b) Identify B: c) Identify D: d) Identify C: Potential Energy
Transcribed image text: Study the following reaction energy diagram: energy reactants products Then answer the following questions about the chemical reaction. Does this reaction release or absorb energy? release absorb neither How many transition states occur during this reaction? 0 yes Could this be an elementary reaction? no If you said this reaction could not be elementary, then how many ...
The following diagram shows a reaction profile. Label the components indicated by the boxes. 1. Total potential energy of the reactants.2. Ea, activation energy of the reaction. This is the difference in energy between the potential energy of the activated complex (transition state) and the potential energy of the reactants.3.
Which of the following diagrams indicates an endothermic reaction? What is the ∆H of that reaction? Diagram 1 is the endothermic reaction. ∆H = 52 kJ. ... your body is burning fuel to give you the energy you need to study. This process heats up the surroundings, causing entropy to increase. The entropy increase of the surroundings offsets ...
Transcribed image text: study the following reaction energy diagram: products energy Then answer the following questions about the chemical reaction. release Does this reaction release or absorb absorb energy? neither How many transition states occur during this reaction? Could this be an elementary reaction? said this reaction could not be elementary, then how many steps are in its mechanism?
95% (19 ratings) 1. The products have higher energy than the reactants. So, the reaction absorbs energ …. View the full answer. Transcribed image text: Study the following reaction energy diagram: products energy reactants Then answer the following questions about the chemical reaction.
34 draw an energy diagram for the following reaction. This preview shows page 6 - 8 out of 8 pages. 1. Show the mechanism for the following elimination, assuming that it is a concerted reaction (E2). I OMe 2. Show the mechanism for the following elimination, assuming that it is a stepwise reaction (E1). Cl OMe 3.
1. Base your answers to the following questions on the energy diagram below. Legend — = uncatatyzed pathway — --- = Cu-catalyzed pathway reaction coordinate a. Does the addition of copper change the rate of reaction? Explain. b. How would increasing the temperature of the uncatalyzed pathway affect the reaction? 6.
Study the following reaction energy diagram: x y 0 reactants energy products Then answer the following questions about the chemical reaction. Question: Study the following reaction energy diagram: x y 0 reactants energy products Then answer the following questions about the chemical reaction.
The energy change in the water bath will be the _____ magnitude in energy as the chemical reaction or phase change, just with the _____. If the water bath gains energy, its temperature goes _____, meaning the energy of the chemical reaction or phase change went down (it lost energy) and vice versa.
Use the following information to answer the next question. Titanium is widely used in the aerospace and sporting goods industries because it is strong, lightweight, and resistant to corrosion. When exposed to oxygen, titanium forms an oxide coating that protects the metal from corrosion. This reaction is represented by the following equation.
All reactions require energy, from the simplest - such as boiling water to turn liquid water into gaseous water - to the complex, such as electrochemical reactions. We can see what is happening to...







:max_bytes(150000):strip_icc()/catalystenergydiagram-56a12b265f9b58b7d0bcb2fe.jpg)









0 Response to "45 study the following reaction energy diagram:"
Post a Comment