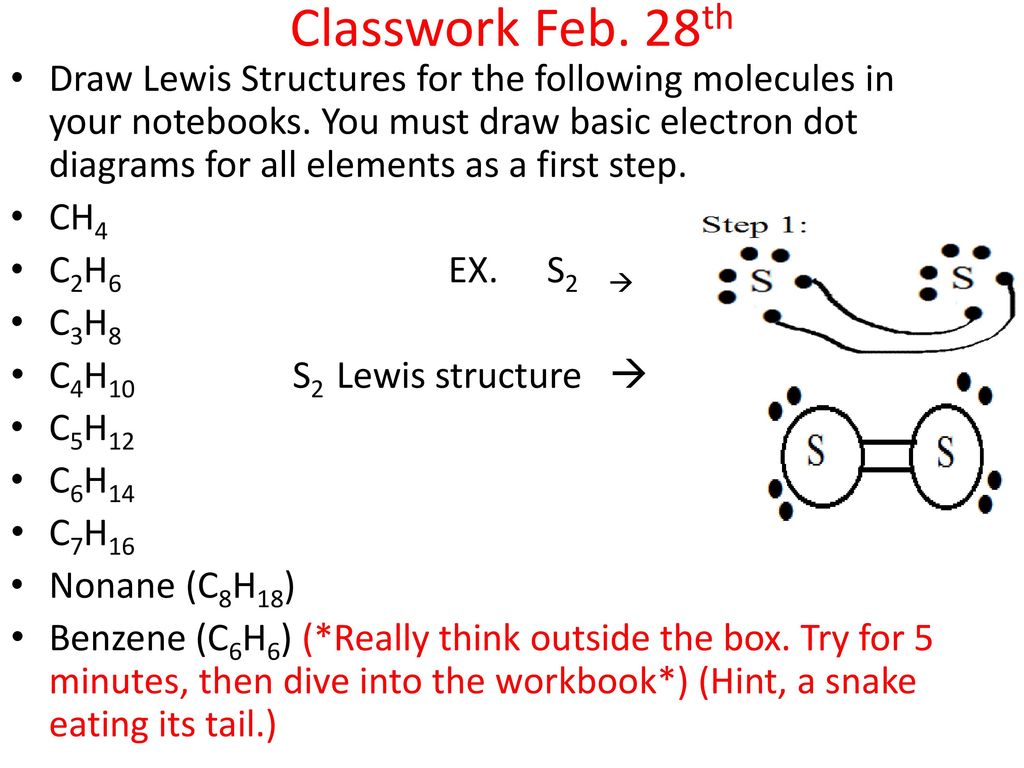
42 ch4 electron dot diagram
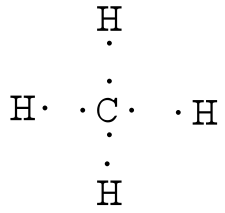
CH4 Lewis Structure - How to Draw the Dot Structure for ... How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use... Ch4 Electron Dot Diagram - schematron.org Lewis dot structure of CH 4. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. C-4 H-1x4=4. Total=8. Put carbon in center and arrange hydrogen atoms on the schematron.orge electrons between carbon and hydrogen atoms. Lewis structures, also known as Lewis dot diagrams ...
NO3 Lewis Structure, Molecular Geometry, and Hybridization 1 day ago · In 1916, American chemist, Gilbert N. Lewis introduced the concept of electron dot structure. Below are some rules to frame any compound’s Lewis dot structure. 1. Follow the octet rule where an atom should complete its outermost shell by the total number of 8 electrons. (Exceptions are hydrogen and boron elements) 2.

Ch4 electron dot diagram
How to Draw the Lewis Dot Structure for CH4: Methane - YouTube A step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use the periodic table to find the total number of vale... MakeTheBrainHappy: The Lewis Dot Structure for CH4 May 18, 2020 - The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the bond has a full valence, with carbon having access to eight electrons and each hydrogen having access to two (this is why hydrogen only ... Lewis structure of CH4:Biochemhelp January 10, 2019 - Step 5: The rest are nonbonding pairs. Subtract bonding electrons (step 3) from valence electrons (step 1). ... Use information from step 4 and 5 to draw the CH4 lewis structure. Alternatively a dot method can be used to draw the CH4 Lewis structure.
Ch4 electron dot diagram. Electron Dot Diagram For Methane - schematron.org Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) . Lewis dot dragram for methane: Methane, with molecular formula CH4, is shown. It is important to remember that Lewis valence dot diagrams are models that Methane is the main component of natural gas, and its chemical formula is CH4. Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ... Draw the electron dot diagram of chemical bonds in methane ... Click here👆to get an answer to your question ️ Draw the electron dot diagram of chemical bonds in methane (CH4) and ethane (C2H6). Draw A Lewis Dot Structure For Ch4 - Novocom.top Draw A Lewis Dot Structure For Ch4, ch4 lewis dot diagram structure structures draw methane electron bonds each ch dots created shown, ch4 lewis dot diagram structure draw molecular estructura hcl methane cross, ch4 dot diagram lewis structure electron methan formel determine lg, dot methane structure electron diagram lewis ch4 diagrams bonding covalent draw bond chemistry hi chemical chem ...
Lewis Dot Structure of CH4 (methane) - YouTube I quickly take you through how to draw the Lewis Structure of methane, CH4. I also go over hybridization, shape and bond angle. CO2 Lewis Structure (2021 UPDATED) All You Need To Know Dec 22, 2021 · In a Molecular Orbital Diagram, the 2s orbital of oxygen is nonbonding because of the high energy difference between carbon and oxygen atoms. Based on the rules of the Lewis Structure, all 16 electrons are filled upon bond formation, but the nonbonding orbitals remain vacant, as in the case of CO2. Lewis Dot Structure for CH4- Methane - Bob Cut Magazine August 12, 2021. Lewis Dot Structure for CH4 (Methane) Properties of methane are described by Lewis Structure as cheaper natural gas than electricity. Methane, or CH4, is a natural gas that is relatively plentiful on earth, making it an environmentally effective source. Because it releases more light and heat when burned, it is more favored for ... What is the correct Lewis dot diagram for CH4 ? - Toppr Do you need help with your homework? On Toppr Answr you can scan any question, and get its answer instantly
41 lewis dot diagram ch4 - Wiring Diagrams Manual Draw the electron dot diagram of CH4 and justify your answer. Medium. Open in App. In CH4 , the central atom is a carbon. In electron dot structure we represent the valence electron of the element. Thus, Carbon has 4 electrons in its electron dot structure and hydrogen has one. methane molecule CH4 Lewis dot & cross electronic diagram covalent... How can the electron dot structure for CH4 be determined ... Answer: I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. I have drawn them above. The red one in the middle is the one Carbon, and the four yellow ones are Hydrogens. Now I have drawn the Valence Electrons. These are the blue dots next to the... Lewis Dot Diagram Ch4 Lewis dot structure of CH 4. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. C-4 H-1x4=4. Total=8. Put carbon in center and arrange hydrogen atoms on the diagramweb.nete electrons between carbon and hydrogen atoms. Dr. draw the electron dot structure of CH4 - Brainly.in November 28, 2017 - Brainly.in is a part of the largest social network for studying in a group. We provide the best tools for mutual help with school subjects. Join us!
Dot Diagram For Ch4 - Wiring Diagrams Lewis dot structure of CH 4. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. C-4 H-1x4=4. Total=8. Put carbon in center and arrange hydrogen atoms on the wiringall.come electrons between carbon and hydrogen atoms.
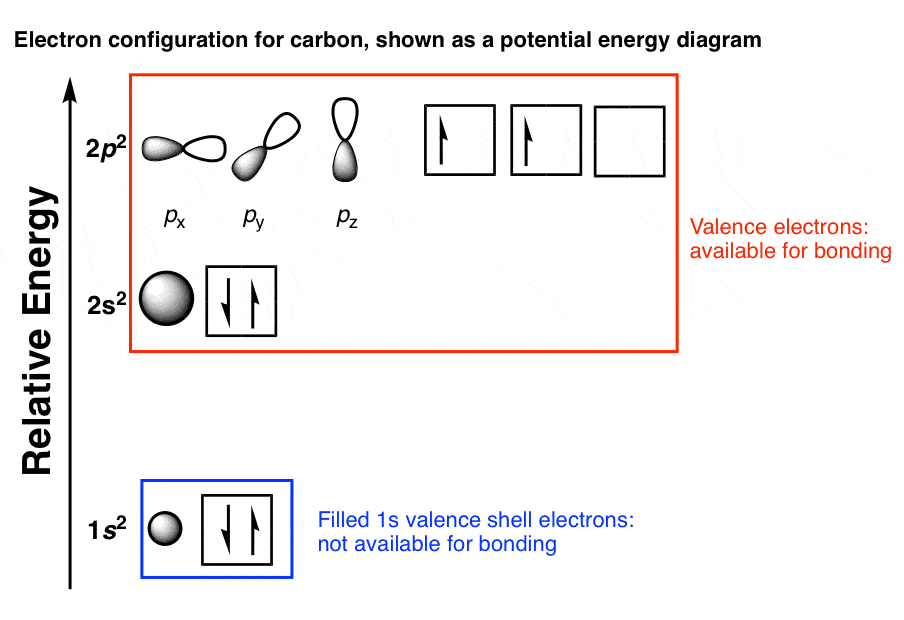
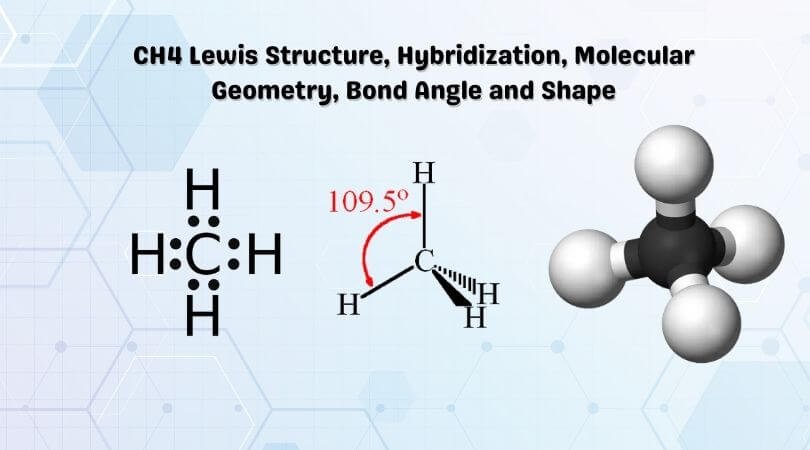
CH4 Lewis Structure, Molecular Geometry, and Hybridization Molecular Orbital diagram of CH4 The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with four 1s atomic orbitals of the hydrogen.
How to draw CH4 Lewis Structure? - Science Education and ... It is represented by dots in the CH4 Lewis diagram. The CH4 molecule's core carbon atom can be represented as follows: To calculate the valence electron of each atom in CH4, look for its periodic group. The carbon and hydrogen families, which are the first and 14th groups in the periodic table, are both made up of carbon and hydrogen astoms.
How to draw the lewis dot structure of Methane (CH4) - YouTube Step by step explanation showing how to draw the Lewis structure of Methane (CH4).
(PDF) Chemistry - Gilbert | Tín Phạm - Academia.edu Academia.edu is a platform for academics to share research papers.
How can the electron dot structure for CH4 be determined? - Quora March 9, 2017 - Answer: I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. I have drawn them above. The red one in the middle is the one Carbon, and the four yellow ones are Hydrogens. Now I have drawn the Valence Electrons. These are the blue dots next to the...
Draw the electron dot diagram of CH4 and justify your answer. Draw the electron dot diagram of CH 4 and justify your answer. Medium Solution Verified by Toppr In CH 4 , the central atom is a carbon. In electron dot structure we represent the valence electron of the element. Thus, Carbon has 4 electrons in its electron dot structure and hydrogen has one. They share electrons to form a C−H single bond.
Lewis Structure Of Ch4 - ViralListClub.com Finding CH 4 molecular geometry using VSEPR theory is not very difficult using these three steps. Lewis structure of CH 4. The Lewis structure of the methane CH4 molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Give the Lewis dot structure of eqCH_4 eq.
CH4 Lewis Structure, Hybridization, Molecular Geometry ... CH4 Lewis Structure Lewis structure is the pictorial representation of the arrangement of valence shell electrons in the molecule, which helps us understand the atoms' bond formations. The electrons that participate in bond formation are called the bonding pair of electrons, while those that don't are known as nonbonding pairs of electrons.
NCERT Solutions for Class 11 Chemistry Chapter 4 - LearnCBSE.in Free NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure solved by expert teachers from latest edition books and as per NCERT (CBSE) guidelines.Class 11 Chemistry Chemical Bonding and Molecular Structure NCERT Solutions and Extra Questions with Solutions to help you to revise complete Syllabus and Score More marks.
Tips for Identifying Intermolecular Forces - Brightstorm An example can be like in Methane, CH4. And so, if I drew a 3D representation of this, where the little dash line here means that this hydrogen is in the back. This hydrogen is in the front, and then this one’s over here. So this would kind of be like a 3D, like a jack that you have here. So let’s take a jack.
CH4 Lewis Structure (Methane) - YouTube Hey Guys,In this video we are going to learn about the Lewis structure of CH4.It is a chemical formula for Methane.To understand the Lewis structure of CH4,w...
CH4 Lewis Structure - How to Draw the Dot Structure for CH4 (Methane) ... How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use...
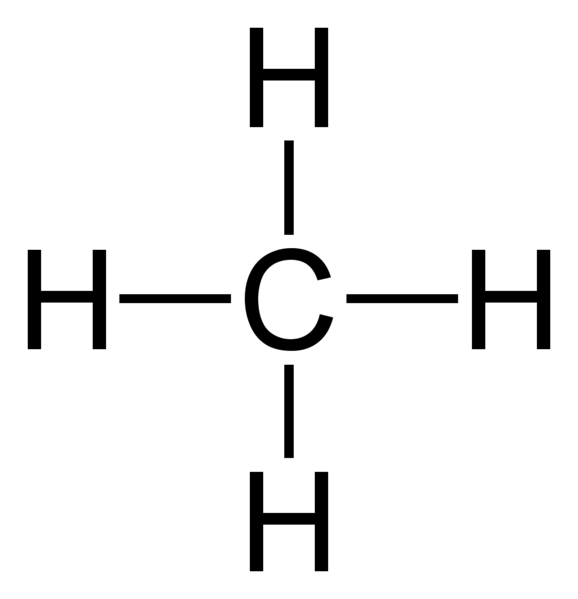
Lewis Dot Structure for CH4 | Chemical Bonding | Electron Dot ... Dr. B. explains how to draw the Lewis dot structure for CH4 (methane). The CH4 Lewis Structure is one of the most frequently tested Lewis Structures · Note that hydrogen atoms always go on the outside of a Lewis dot structure. This is because they can share a maximum of two electrons.
Lewis structure calculator | Lewis structure generator The Lewis Structure Generator that we put in your hands here is an excellent tool to obtain structures of more than 400 molecules. To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it.
What is the Lewis dot structure for CH4? | Study.com In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
How to Draw the Lewis Structure of CH4 (methane) - YouTube Check me out:
Ch4 Lewis Diagram - ch4 molecular geometry shape and bond ... explain the bonding in methane molecule using electron dot. Ch4 Lewis Diagram. Here are a number of highest rated Ch4 Lewis Diagram pictures upon internet. We identified it from trustworthy source. Its submitted by organization in the best field. We give a positive response this kind of Ch4 Lewis Diagram graphic could possibly be the most ...
Ch4 Lewis Structure Drawing - Novocom.top ch4 lewis dot diagram covalent study compounds bonding chemical structure correct which structures chemistry electrons atom chapter exam holt question . structure ch4 lewis dot methane tetrahedral shape 3d geometry molecule dimensional electron shapes molecules three h2s structures sundin nh3 bond .
Draw electron dot structure of methane and ethane - Brainly.in February 3, 2018 - Brainly.in is a part of the largest social network for studying in a group. We provide the best tools for mutual help with school subjects. Join us!
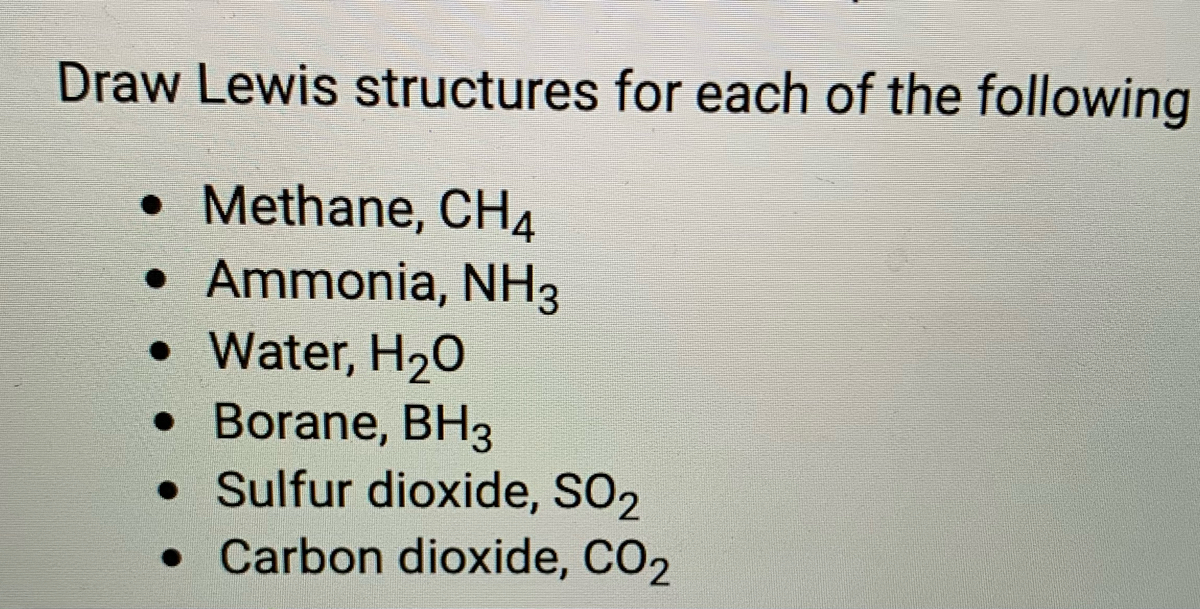
Lewis Dot Structures 1. Methane 4 - HCC Learning Web Lewis Dot Structures 1. Methane - CH 4 Number of Valence Electrons: 4 from C and 1 each from 4 H = 8 Carbon is more electronegative than hydrogen, but hydrogen can never be the "central" atom, as it can only form 1 bond. Carbon always forms 4 bonds (2 electrons each). 2. Ammonia - NH 3
Draw the electron - dot structure of CO2 - Toppr Ask In an IIT (F) class a teacher asked the students to sketch the potential energy diagram for the formation of hydrogen molecule. Four students A, B, C and D drew the following diagrams a, b, c and d among which three were considered wrong and one correct by the teacher. Guess the correct potential energy diagram and justify it. The atomic number ...
VSEPR The calculation for methane shows that the carbon atom is associated with 8 electrons in the σ framework. This corresponds to four shape-determining electron pairs. The coordination geometry of carbon is consequently tetrahedral. There are four bonded groups, therefore there are no lone pairs ...
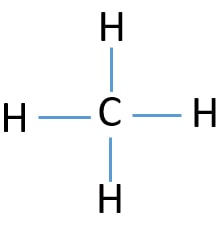
Lewis Structure for CH4 (Methane) - UMD Drawing the Lewis Structure for CH 4. For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single bonds.It's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.
Draw the electron dot structure of CH4. Methane is a gas made up of one carbon atom for every four hydrogen atoms. The four hydrogen atoms around the core carbon mean that there are four groups of electrons around this carbon, giving the molecule a tetrahedral form. The core element in CH4 is carbon. The valence electron of the element is ...
Draw the electron dot diagram of CH4 and justify your answer. Do you need help with your homework? On Toppr Answr you can scan any question, and get its answer instantly
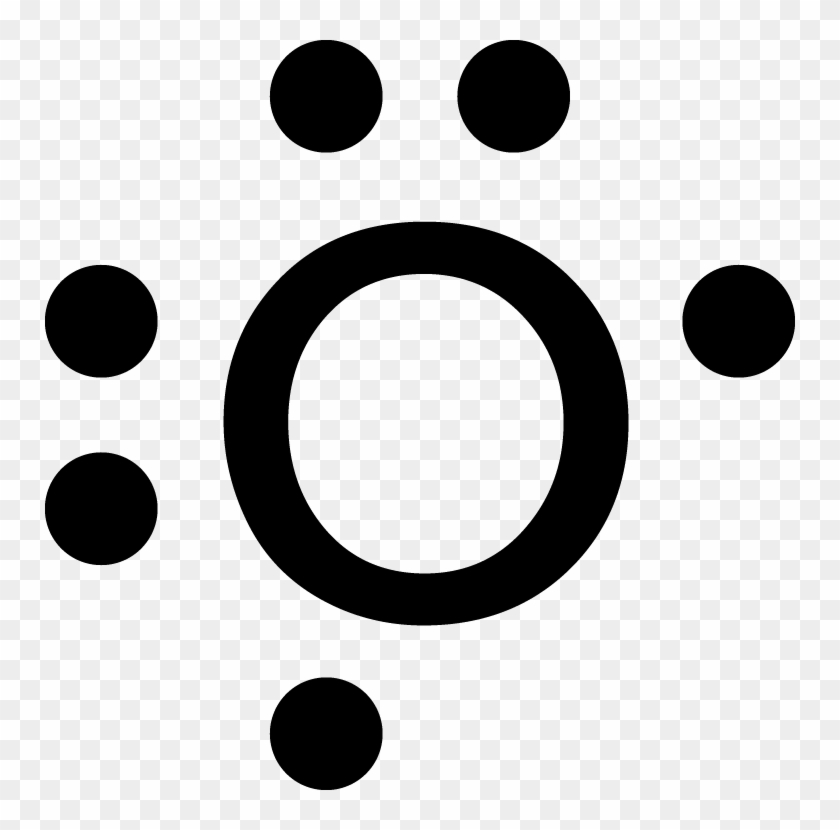
Dot Diagram For Ch4 - Wiring Diagram Pictures Lewis Dot Structure for CH4 #2 Find the number of "octet" electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 .May 03, · A step-by-step explanation of how to draw the Lewis Structure Oxygen Gas (Dioxygen). For the O2 Lewis structure, calculate the total number of valence electrons for the ...
Carbon tetrabromide (CBr4) lewis dot structure, molecular ... Follow some steps for drawing the lewis dot structure of CBr4. 1. Count total valence electron in CBr4. Finding the total number of valence electrons in the CBr4 molecule is the first step for drawing its lewis diagram. “A valence electron is the outermost shell electrons around an atom”.
Ch4 Electron Dot Diagram The electrons are arranged in four pairs representing four covalent bonds. The Lewis dot structure for CH 4 shows the number of valence electrons around each atom. Each dot represents a valence electron. The number of valence electrons . Write the symbol of the atom you are drawing the electron dot diagram for in the middle of your paper.
How do you draw the electron dot structure for CH4? - Answers Some possible way to show the structure of CH4 are its electron dot diagram or structural formula. CH4 or methane's molecular formula is given as CH4. The structural formula is a graphical...
Draw Lewis dot diagram for the following. Methane (CH4) Diagram. Draw Lewis dot diagram for the following. Methane (CH 4) Advertisement Remove all ads.
Draw the electron dot diagram of CH4 and justify your answer. Click here to get an answer to your question ✍️ Draw the electron dot diagram of CH4 and justify your answer.
NCERT Exemplar Class 10 Science Solutions Chapter 4 - BYJUS 41. In electron dot structure, the valence shell electrons are represented by crosses or dots. (a) The atomic number of chlorine is 17. Write its electronic configuration (b) Draw the electron dot structure of chlorine molecule. Soln: KLM- 2,8,7; 42. Catenation is the ability of an atom to form bonds with other atoms of the same element.
CH4 lewis structure, Molecular geometry, Polar or nonpolar ... The molecular geometry or shape for CH4 is the tetrahedral with bond angle ∠H−C−H =109.5°. The electron geometry for CH4 is also tetrahedral as it central has 4 regions of electron density with no lone pair on it. Lewis dot structure of CH4 contains only 4 bonded pairs (8 shared electrons) and doesn't contain any lone pair electrons in ...
How to determine the Lewis dot structure for methane - Quora June 17, 2017 - Answer: Well Carbon only has 4 valence electron, so it can bond at all four point. Hydrogen only has one valence electron and can only share one. Methane’s (When it comes to hydrocarbons, “meth” stipulates one carbon, “ane” stipulates a single bond shared with hydrogens) molecular ...
Lewis structure of CH4:Biochemhelp January 10, 2019 - Step 5: The rest are nonbonding pairs. Subtract bonding electrons (step 3) from valence electrons (step 1). ... Use information from step 4 and 5 to draw the CH4 lewis structure. Alternatively a dot method can be used to draw the CH4 Lewis structure.
MakeTheBrainHappy: The Lewis Dot Structure for CH4 May 18, 2020 - The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the bond has a full valence, with carbon having access to eight electrons and each hydrogen having access to two (this is why hydrogen only ...
How to Draw the Lewis Dot Structure for CH4: Methane - YouTube A step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use the periodic table to find the total number of vale...



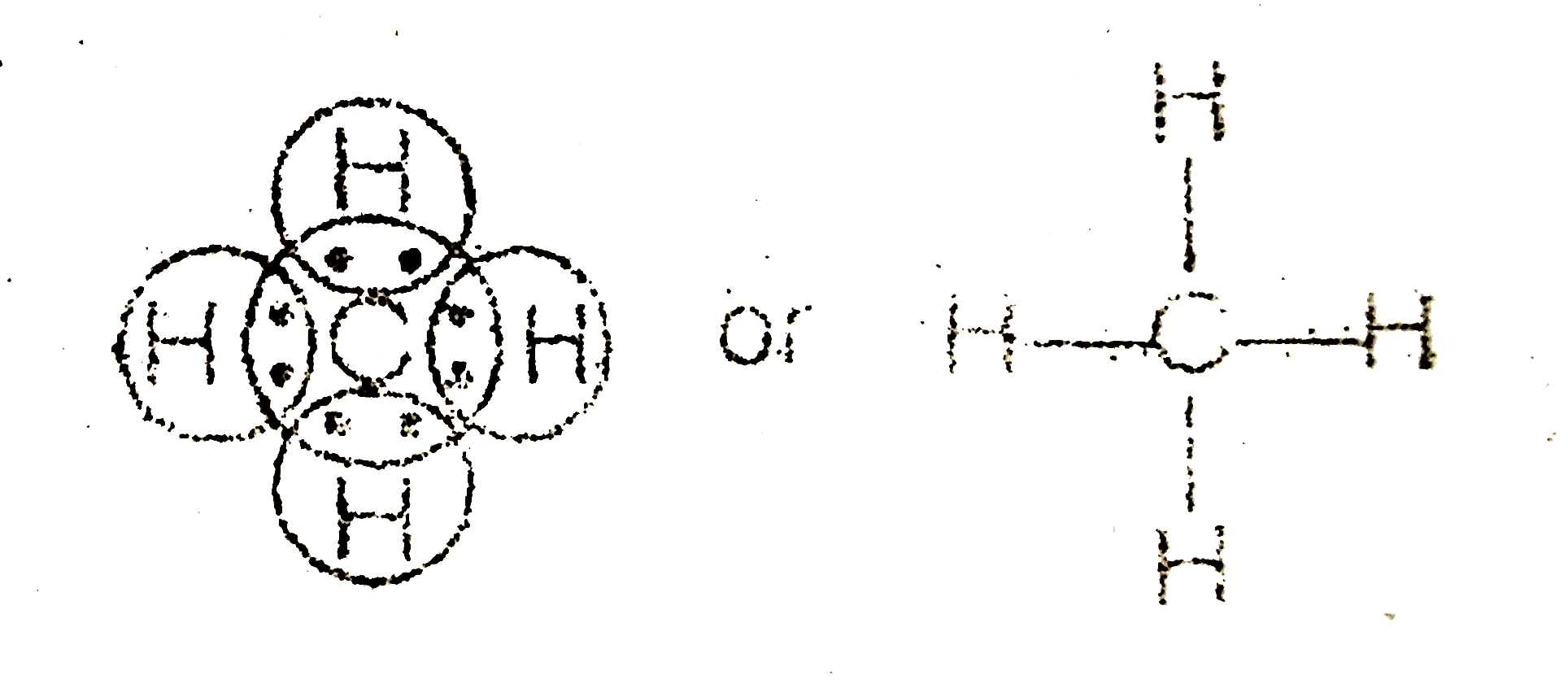



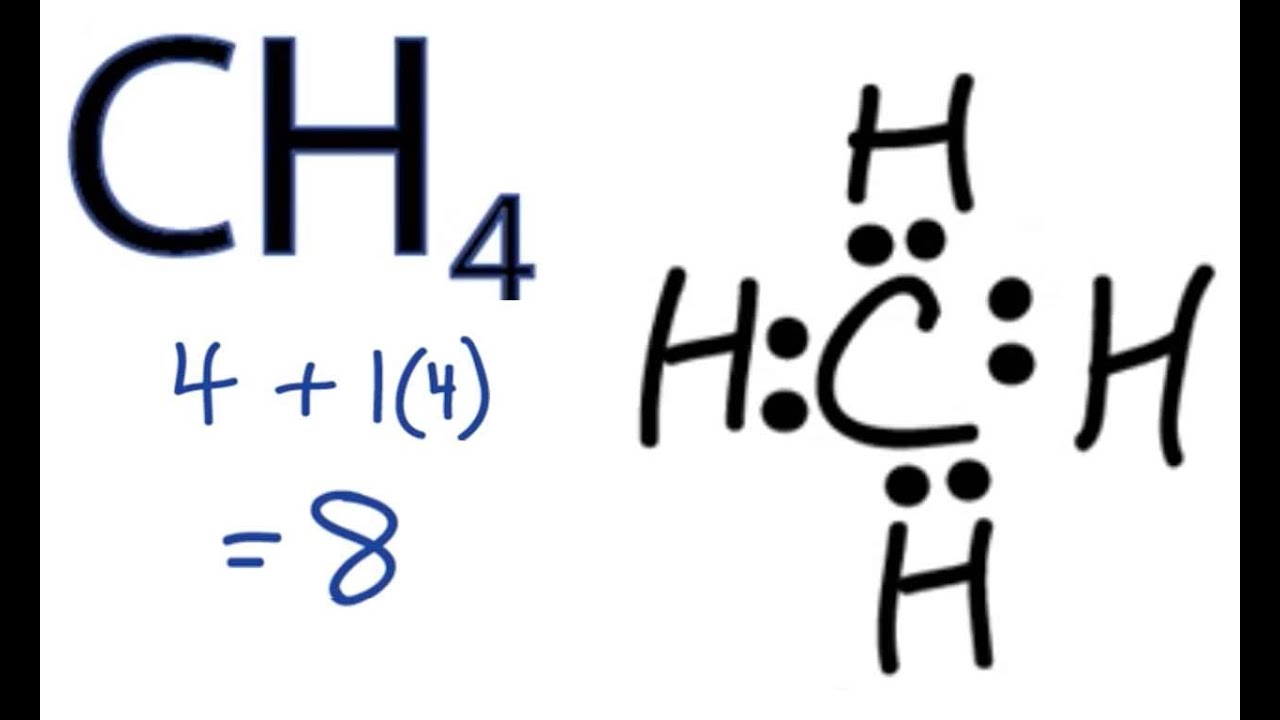




















0 Response to "42 ch4 electron dot diagram"
Post a Comment