45 molecular orbital diagram for water
Molecular orbital diagram - Wikipedia Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. Molecular orbital diagram - WikiMili, The Best Wikipedia ... A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. [1] [2] [3] A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the ...
Molecular Orbital Theory - Purdue University Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...

Molecular orbital diagram for water
How many molecular orbitals are in H2O ... Water has 4 regions of electron density around the central oxygen atom (2 bonds and 2 lone pairs). These are arranged in a tetrahedral shape. The resulting molecular shape is bent with an H-O-H angle of 104.5°. What is water molecule diagram? A water molecule consists of two hydrogen atoms and one oxygen atom. 7.7 Molecular Orbital Theory - Chemistry Fundamentals Molecular Orbital Energy Diagrams. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. PDF Inorganic Chemistry with Doc M. - Creighton University 2. The 10-Step approach to making MO diagrams via symmetry considerations 3. Molecular orbital diagram for bent H2O 4. Molecular orbital diagram for linear BeH2 5. Molecular orbital diagram for square planer XeF4 6. Using p-orbitals for σ-bonding: molecular orbital diagram for trigonal planer BF3 1.
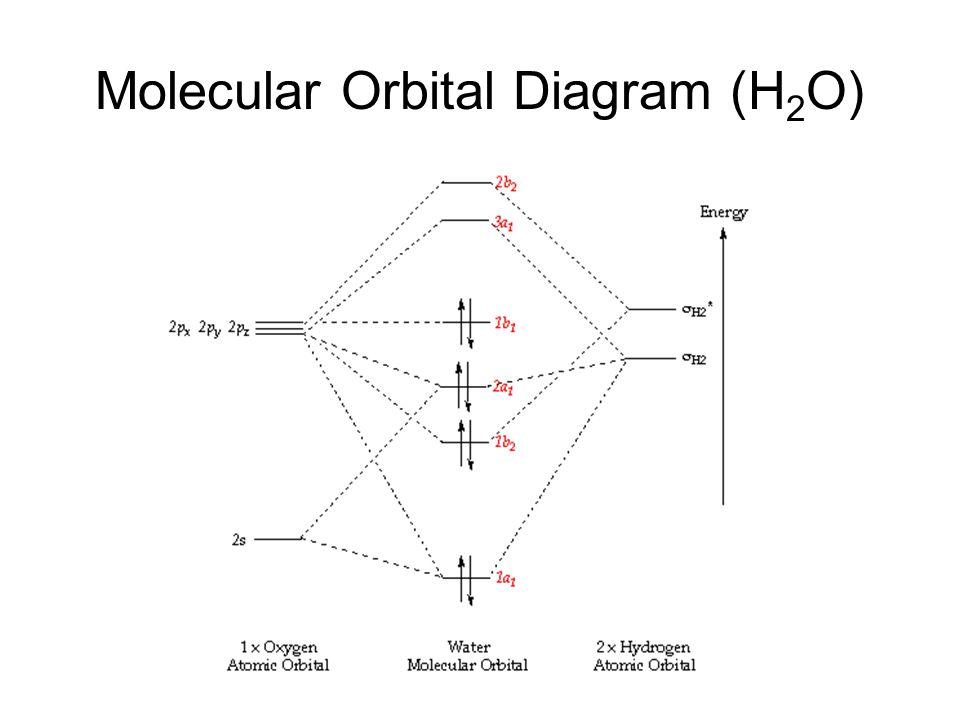
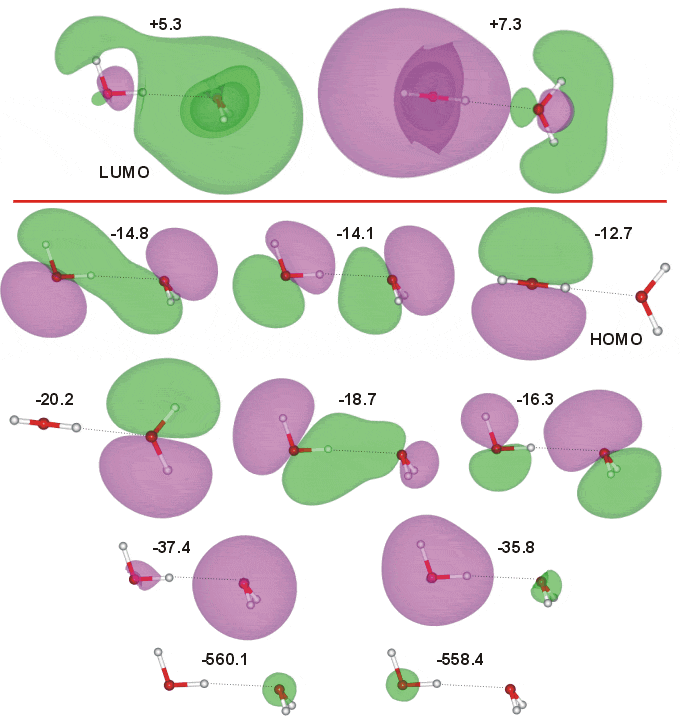
Molecular orbital diagram for water. Molecular orbitals for water (H2O) Molecular Orbitals for Water (H 2 O). The five occupied and the lowest three unoccupied molecular orbitals of the isolated molecule (1a 1) 2 (2a 1) 2 (1b 2) 2 (3a 1) 2 (1b 1) 2 (4a 1) 0 (2b 2) 0 (3b 2) 0 were calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set. b (experimental data is given in []).They are set out with the lowest energy (that is, most ... What is the 3d structure of H2O? - Easierwithpractice.com Molecular Orbital diagram of water (H2O) The molecular orbital diagram is a pictorial representation of determining chemical bonding between the molecules of a compound. Furthermore, the molecular orbital diagram helps with determining how two sigma bonds have been formed and the effect of the lone pairs on the structure. What is the molecular orbital diagram of co2? - idswater.com What is the molecular orbital diagram of co2? The carbon dioxide MO diagram is based on a C atom and an O-O ligand fragment. Carbon has 2S and 2Px,y,z orbitals and the O-O fragment has 2S and 2Px,y,z orbitals that are involved in the formation of molecular orbitals. Molecular orbital diagram - Infogalactic: the planetary ... Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
PDF Molecular Orbitals for Water (H2O) - idc-online.com orbital in the gas phase is 539.9 eV [1227]. These orbitals are appreciably changed in ice and water; the experimental electron binding energies in liquid water being 2a 1 30.90 eV, 1b 2 17.34 eV, 3a 1 13.50 eV, 1b 1 11.16 eV [877]. The experimental binding energy of the 1a 1 orbital in the liquid phase consists of a broad energy distribution ... EXP 6 Molecular Orbitals of Water .pdf - 6.1 Experiment 6 ... Molecular orbital energy level diagram for H 2 O. Moving to a triatomic molecule, such as water, can complicate things further, given that an energy diagram would now need to be "three-sided" since three atoms are now involved. In the case of water, the simpler "two-sided" diagram can still be used, if we treat the two H 1s orbitals together. H2O Lewis Structure, Molecular Geometry, and Hybridization Molecular Orbital diagram of water (H2O) The molecular orbital diagram is a pictorial representation of determining chemical bonding between the molecules of a compound. Furthermore, the molecular orbital diagram helps with determining how two sigma bonds have been formed and the effect of the lone pairs on the structure. molecular orbital diagram : OrganicChemistry It works, as you can easily find mo diagrams for water (and I think F3- as well). When atoms are different, the line between bonding, non-bonding and anti-bonding is not that clear, as you need to compare the new orbital's energy and shape compared to the original orbitals...
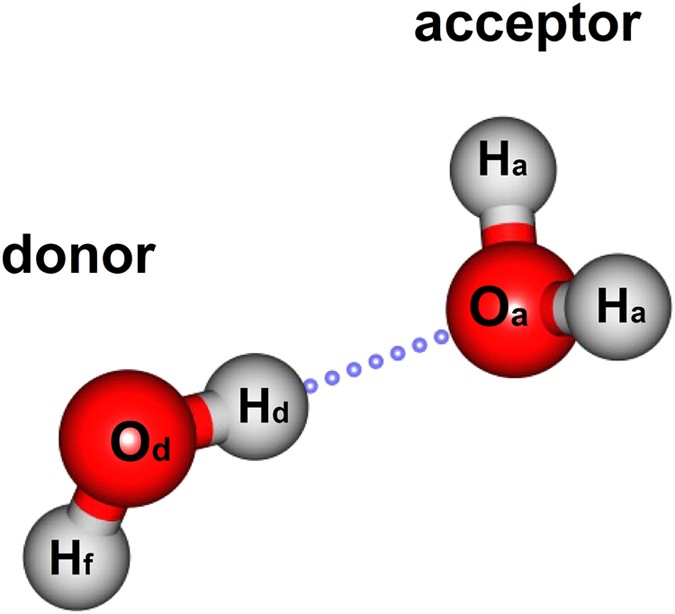
Molecular orbital analysis of the hydrogen bonded water ... Figure 2: The orbital interaction diagram of (H2O)2. Orbital energy levels are represented as solid bars. The bars on the left and right sides correspond to the FOs of the two water monomers; the... Molecular Orbital Theory - Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in . Molecular Orbitals - Molecular Orbitals for Polyatomic ... In summary, the electronic configuration of the water molecule as determined by molecular orbital theory is 1a 21 2a 21 1b 22 3a 21 1b 21 The la 1 orbital is a nonbinding inner shell orbital. The pair of electrons in the la 1 orbital simply screen two of the nuclear charges on the oxygen from the protons. Empirical & semi-empirical MO theory - MOs for water and ... Hydrogen orbitals These may be combined to form the molecular orbitals of water: This diagram illustrates an important point - the conservation of orbitals , i.e. if you start with six AOs, you must end up with exactly six MOs, and there must be the same number of each symmetry.
Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration.
PDF Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules 4 Lecture 2 Pi bond (π): bonding molecular orbital -The bonding electron density lies above and below, or in front and in back of the bonding axis, with no electron directly on the bonding axis, since 2p orbitals do not have any electron density at the nucleus.
Introduction to Molecular Orbital Theory The atomic orbitals combine to produce the following molecular orbital diagram: Here the 2 p g orbital is occupied by two electrons to give a total bond order of three. This corresponds well with the Lewis structure ( ), although the orbital approach tells us that there is one s and two p .
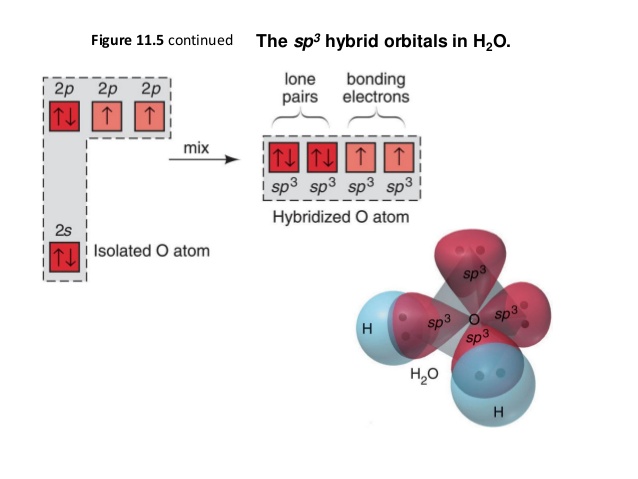
Chemical bonding of water - Wikipedia Hybridized Molecular Orbital (MO) diagram of H 2 O. To further distinguish the electron energy differences between the two non-bonding orbitals, orbital mixing can be further performed between the 2p (3a 1) orbital on oxygen and the antibonding 4a 1 orbital since they are of the same symmetry and close in energy level. Mixing these two orbitals ...
Solved Report Sheet: Molecular Orbitals of Water 1. Match ... Water ( H 2 O) is an isolated molecule having five occupied and three unocc …. View the full answer. Transcribed image text: Report Sheet: Molecular Orbitals of Water 1. Match the orbital designations (1a, 2a, 3a, 1b, 1b, or 2b,) with each of the following possible combinations of atomic orbitals: a. 0+H +H. Orbital Designation b. 0:p+ H+H.
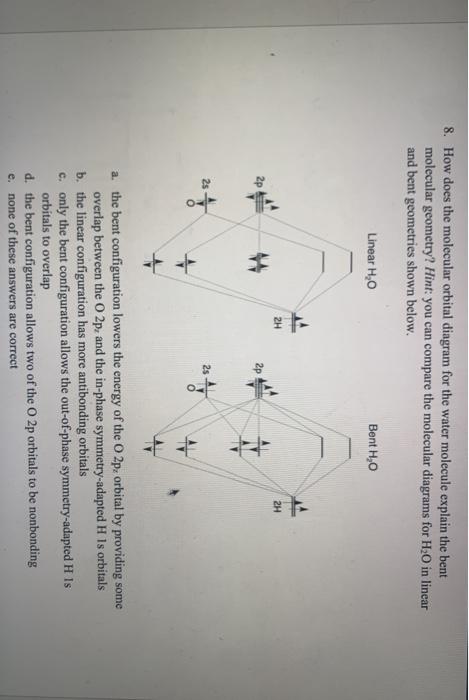
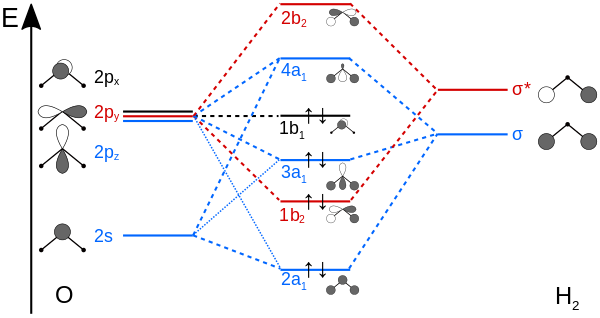
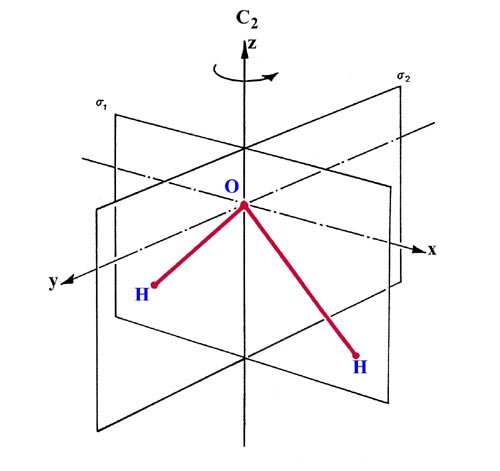
PDF Polyatomic Molecular Orbital Theory - La Salle University Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H
PDF Hybrid Molecular Orbitals - University of Illinois Urbana ... 6. There is one p orbital on boron but there is no adjacent atom with another p orbital. Add it to the molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1.
H20 Molecular Orbital Diagram - schematron.org A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Chemical bonding of H2O - WikipediaChemical bonding of H2O - Wikipedia
PDF MO Diagrams for Linear and Bent Molecules Water With the orbital shapes, symmetries, and energies in hand we can make the MO diagram! A1 A1 B1 B2 -15.8 eV -32.4 eV A1 B1 -13.6 eV 2a1 3a1 4a1 1b1 2b1 1b2 nb nb σ σ Two bonds, two lone pairs on O. HOMO is nonbonding.
H20 Molecular Orbital Diagram - Wiring Diagrams H20 Molecular Orbital Diagram. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals. General procedure for simple molecules that contain a central atom: build group orbitals using the outer atoms, then interact the group orbitals.
Molecular Orbital (MO) Diagram of Polyatomic molecules ... In polyatomic molecules we can have more than two atoms combining, e.g. in case of beryllium hydride there are 3 atoms overlapping simultaneously. So in this...
PDF Inorganic Chemistry with Doc M. - Creighton University 2. The 10-Step approach to making MO diagrams via symmetry considerations 3. Molecular orbital diagram for bent H2O 4. Molecular orbital diagram for linear BeH2 5. Molecular orbital diagram for square planer XeF4 6. Using p-orbitals for σ-bonding: molecular orbital diagram for trigonal planer BF3 1.
7.7 Molecular Orbital Theory - Chemistry Fundamentals Molecular Orbital Energy Diagrams. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right.
How many molecular orbitals are in H2O ... Water has 4 regions of electron density around the central oxygen atom (2 bonds and 2 lone pairs). These are arranged in a tetrahedral shape. The resulting molecular shape is bent with an H-O-H angle of 104.5°. What is water molecule diagram? A water molecule consists of two hydrogen atoms and one oxygen atom.






















![Conceptual MO-diagram of [Co(II/III)(L1) 3 ] 2+/3+ in high ...](https://www.researchgate.net/profile/Chunzhen-Yang/publication/322841939/figure/fig1/AS:589124784390144@1517469702597/Conceptual-MO-diagram-of-CoII-IIIL1-3-2-3-in-high-spin-and-low-spin-states-For.png)



0 Response to "45 molecular orbital diagram for water"
Post a Comment