43 silicon electron dot diagram
SiO2 Lewis Structure| Step By Step Construction - What's Insight SiO2 Lewis Structure (Step by Step Construction) In the SiO 2 lewis Structure, the overall ratio of silicon to the oxygen atom is 1:2.The Silicon oxygen bonds are strong and keep the atoms firmly in place. Following are the steps to construct the SiO 2 Lewis Structure.. Step-1: Count the valence electrons of atoms For the SiO 2 Lewis structure, we need to figure out the number of valence ... Drawing Lewis diagrams (video) - Khan Academy And then the silicon is able to share in four bonds. Each of those bonds have two electrons, so the silicon is also feeling good about the octet rule. So I would feel very confident in this being the Lewis diagram, sometimes called the Lewis structure, for silicon tetrafluoride.
Lewis Dot Diagram For Silicon - Summarized by Plex.page | Content ... The SiO 2 Lewis Structure was constructed in the following steps. For the SiO 2 Lewis structure, we need to determine the number of valence electrons in individual atoms as shown in the Table. VEs in SiO 2 = VEs in 1 Silicon atom + VEs in 2 Oxygen atoms Silicon dioxide also known as silica is the most common and most important component of ...

Silicon electron dot diagram
What is the Lewis dot structure for silicon? How is it ... Silicon is in Group 14 (sometimes called Group IV or 4). Since it is in Group 4 it will have 4 valence electrons. When you draw the Lewis structure for Silicon ...2 answers · 1 vote: Apparently there are different schools of thought on this (How to Draw Electron Dot Diagrams ... Electron Configuration for Silicon (Si) - UMD Therefore the Silicon electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 2. Video: Silicon Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to ... SiO2 Lewis Structure: How to Draw the Dot Structure for SiO2 | Chemical ... Let's do the SiO2 Lewis structure. On the periodic table, Si is in group 4, it has 4 valence electrons. Oxygen has 6, but we have two Oxygens, for a total of 16 valence electrons. We'll put the Si in the center and then the Oxygens on either side. We'll put two electrons between atoms to form bonds, and the rest around the outside atoms.
Silicon electron dot diagram. ⚗️Which model represents the electron dot diagram of silicon? - Brainly.com Which model represents the electron dot diagram of silicon? 2 See answers Advertisement Advertisement jasonthegod01 jasonthegod01 Answer: b. Explanation: im pretty sure could be wrong. tysm it was right Thank you it was right!! Advertisement Advertisement reptilelover2352 reptilelover2352 Silicon Oxide Lewis Dot Structure | Science | ShowMe 0 people liked this ShowMe. Flag ShowMe. Viewed after searching for: Lewis electron dot structure calcium hydride. Lewis electron dot structure Mg. cacl2 lewis structure. Lewis dot Structure of mgcl2. Lewis dot structure for Al2O3. You must be logged into ShowMe. SiS2 Lewis Structure, Molecular Geometry ... - Techiescientist Lewis structure of Silicon disulfide (SiS2) Before studying the Lewis structure of Silicon disulfide, it is crucial to analyze the Lewis structures of participating atoms which are Silicon and Sulfur. The atomic number of Silicon is 14, and the electronic configuration is 1s2 2s2 2p6 3s2 3p2. silicon tetrachloride dot and cross diagram Germanium tetrachloride is an intermediate for the purification of germanium metal or its oxide, GeO 2. A lewis electron dot diagram or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Hydrogen can only form 1 bond..
What is the electron dot structure for silicon? - Answers There are four valance electrons in Silicon, therefore there will be 4 dots in your electron dot diagram. The Lewis structure for silicon disulfide is to be predicted. Concept ... The Lewis structure for silicon disulfide is to be predicted. Concept introduction: The strategy for drawing Lewis structure is mention below. Calculate the number of valence electrons present in the molecule. Calculate the electron pairs by diving number of valence electrons by 2. Determine the bond pairs. Determine the lone pairs. Lewis Electron Dot Structures - Detailed Explanation with Examples & Videos Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. Silicon tetraiodide | SiI4 - PubChem Silicon tetraiodide | SiI4 or I4Si | CID 83498 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological ...
Silicon tetraiodide - Wikipedia Silicon tetraiodide is the chemical compound with the formula Si I 4. It is a tetrahedral molecule with Si-I bond lengths of 2.432 (5) Å. SiI 4 is a precursor to silicon amides of the formula Si (NR 2) 4 (R = alkyl). It has also been of interest in the manufacture and etching of silicon in microelectronics . What is the Lewis dot structure for silicon? How is it determined ... Answer (1 of 2): Apparently there are different schools of thought on this (How to Draw Electron Dot Diagrams). That said, it seems that the one followed (at least in the Western US) has you start with the right hand side and the S orbital (only the outer valence electrons!): Si: (easier to do t... SiO2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity ... Conclusion. SiO2 has a net dipole moment of zero. It has a linear electron and molecular geometry with a bond angle of 180 degrees and a hybridization of Sp. The Silicon dioxide Lewis structure has a total of 16 valence electrons. In the Lewis dot structure of SiO2, the formal charge is zero. What is the electron dot diagram for Helium? - Qaalot What is the electron dot diagram for Helium? Due to this fact Helium solely had 2 valence electrons. It is positioned in Group 8A as a result of it is outer shell is full with two electrons. While you draw the Lewis construction for Helium you may put two "dots" or valance electrons round the aspect image (He). Click on to see full reply.
electron dot structure of silicon - Brainly.in
Silicon carbide | SiC - PubChem Silicon carbide | SiC or CSi | CID 9863 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities ...
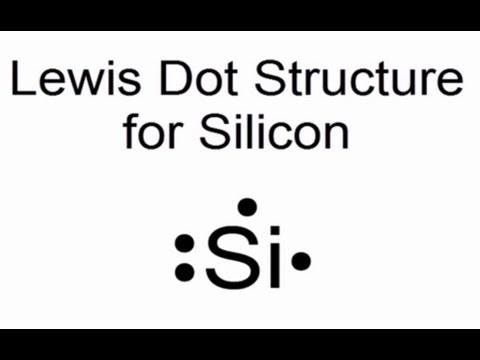
Silicon Bohr Model - How to draw Bohr diagram for Silicon (Si) atom Electron dot diagram of a Silicon atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Silicon, we got to know, it has only 4 valence electrons. So, just represent the 4 valence electrons around the Silicon atom as a dot.
What Is The Electron Dot Structure Of Silicon? [Comprehensive Answer] The symbol in an Electron Dot Diagram is used to represent the nucleus and all the non-valence electrons, The Electron Dot Diagram is also known as the Lewis Dot Symbol. The Lewis Dot Symbol or Electron Dot Diagram, is a symbol of an element with one or more dots representing the valence electrons of an element.
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
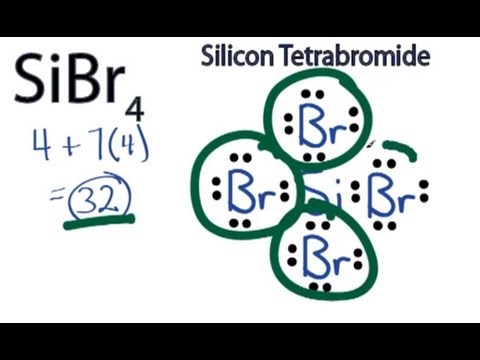
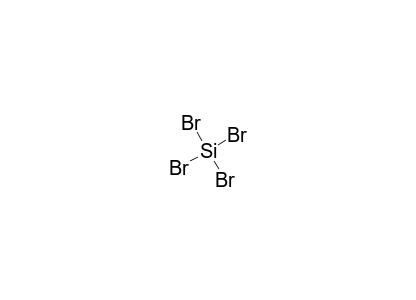
SiCl4 Lewis structure, Molecular geometry, Bond angle, Polarity, Electrons Summary. The total valence electron is available for the Silicon tetrachloride (SiCl4) lewis structure is 32. The hybridization of the SiCl4 molecule is Sp 3. The bond angle of SiCl4 is 109.5º. SiCl4 is nonpolar in nature, although, its bonds are polar. The overall formal charge in Silicon tetrachloride is zero.
Lewis Dot Structure for Silicon Atom (Si) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for Si (Silicon). I show you where Silicon is on the periodic table and how to determine h...
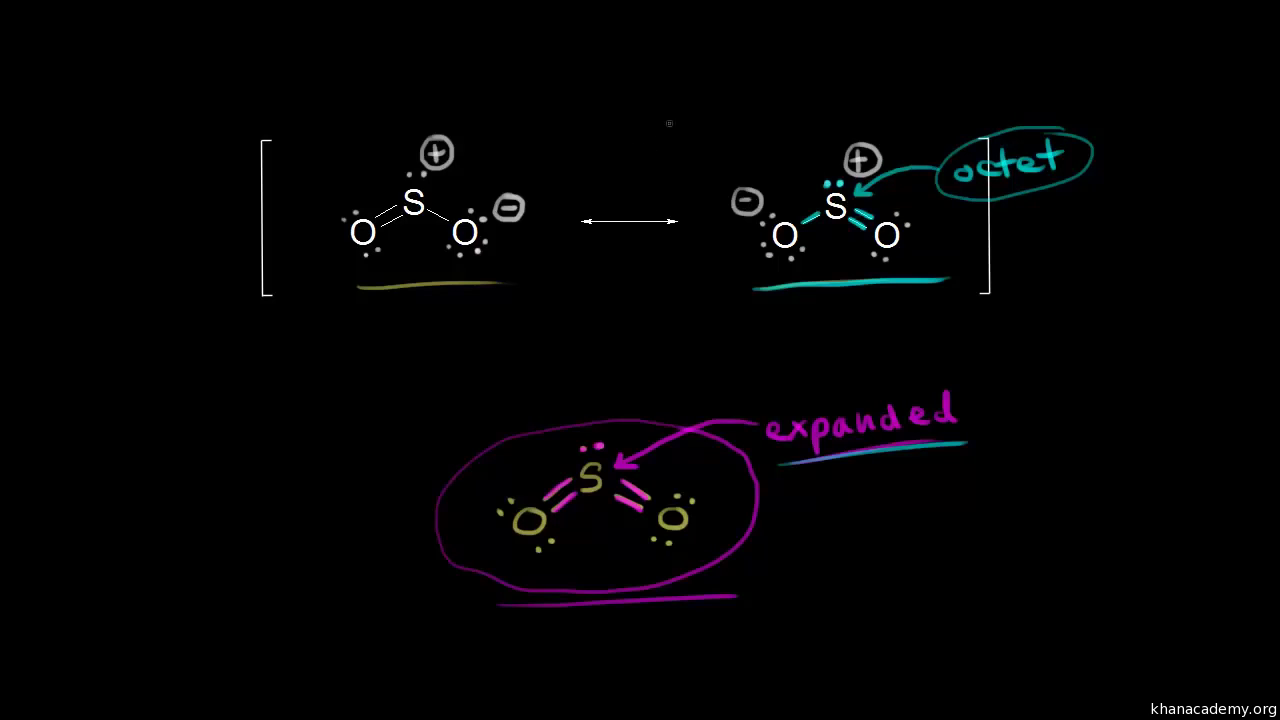
Lewis Structures ... 100+ Lewis Structures - The Geoexchange Check the Formal Charges to make sure you have the best Lewis Structure. Explain How Examples: SO 4 2-, N 2 O, XeO 3; Notable Exceptions to the Octet Rule. H only needs 2 valence electrons. Be and B don't need 8 valence electrons. S and P sometimes have more than 8 val. Electrons.
Silicon Electron Dot Diagram - mungfali.com Electron Dot Diagram For Sodium - Wiring Site Resource. Electron Dot Diagram For Silicon - Free Wiring Diagram. =章 9 Section A Lewis Electron Dot Diagrams
Silicon: Element Lewis Structure, Facts & Discovery - Study.com Silicon is in group 14 and period 3 of the periodic table and has four valence electrons in its Lewis structure. The four valence electrons means that silicon can bond in a way similar to carbon ...
Lewis Dot Diagram For Tellurium - schematron.org Write the electron dot (Lewis) diagrams for the following. 9. carbon silicon Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol.
SiH4 Lewis Structure - How to Draw the Lewis Structure for ... - YouTube A step-by-step explanation of how to draw the SiH4 Lewis Dot Structure (Silicon Tetrahydride).For the SiH4 structure use the periodic table to find the total...
SiO2 Lewis Structure: How to Draw the Dot Structure for SiO2 | Chemical ... Let's do the SiO2 Lewis structure. On the periodic table, Si is in group 4, it has 4 valence electrons. Oxygen has 6, but we have two Oxygens, for a total of 16 valence electrons. We'll put the Si in the center and then the Oxygens on either side. We'll put two electrons between atoms to form bonds, and the rest around the outside atoms.
Electron Configuration for Silicon (Si) - UMD Therefore the Silicon electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 2. Video: Silicon Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to ...
What is the Lewis dot structure for silicon? How is it ... Silicon is in Group 14 (sometimes called Group IV or 4). Since it is in Group 4 it will have 4 valence electrons. When you draw the Lewis structure for Silicon ...2 answers · 1 vote: Apparently there are different schools of thought on this (How to Draw Electron Dot Diagrams ...





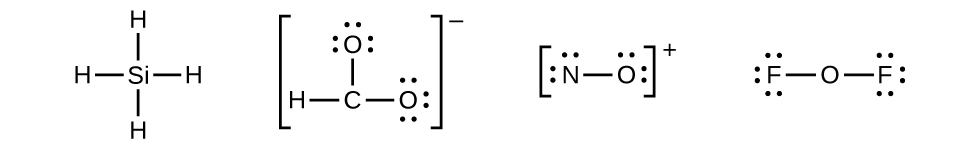




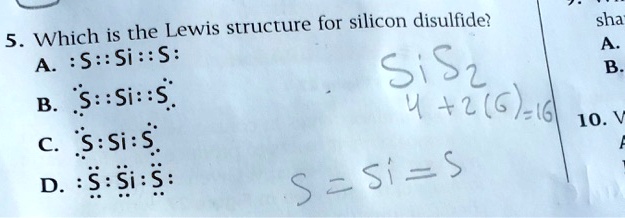







0 Response to "43 silicon electron dot diagram"
Post a Comment