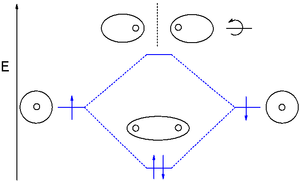
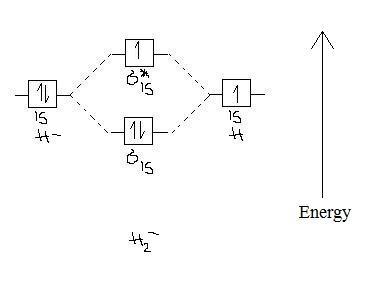
41 use the mo diagram given to find the bond order for h2-.
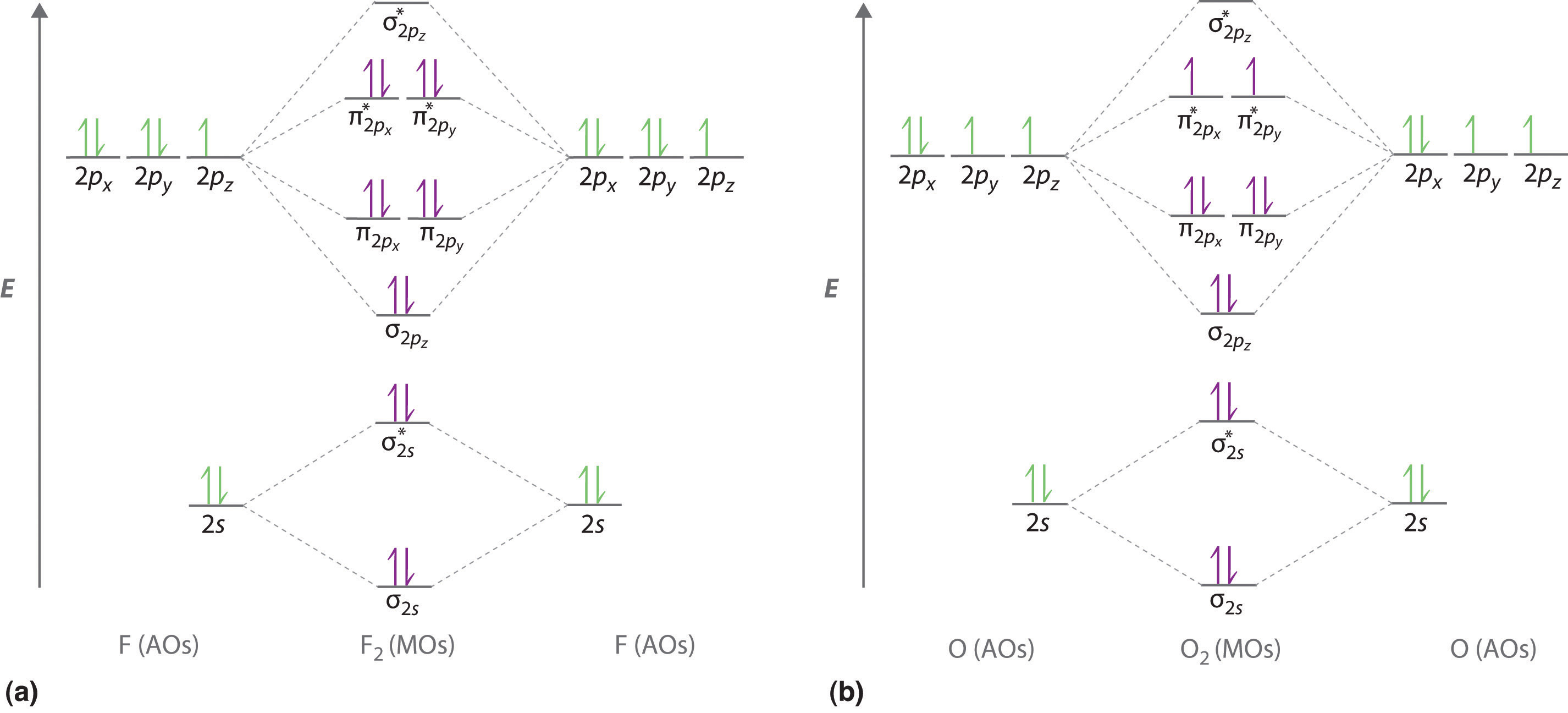
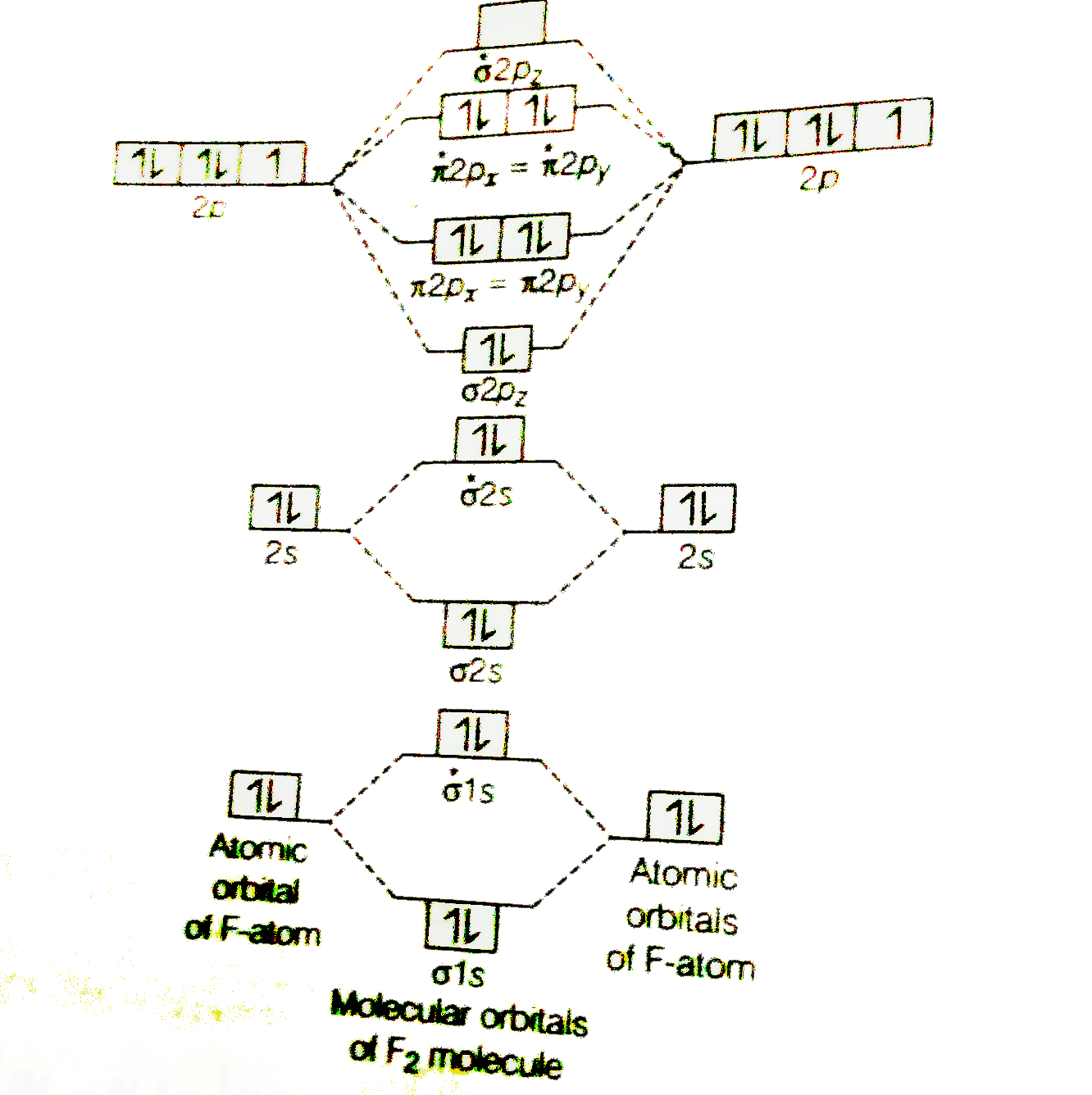
Click here👆to get an answer to your question ️ 37. Draw molecular orbital diagram for F2 molecule. Also, give its electronic configuration, bond order and magnetic property. 138. Solve the following: 3:31For the ion H2+:a) Draw the molecular orbital diagram.b) Calculate the bond order.c) Would this ion exist ...2 Nov 2012 · Uploaded by Professor Heath's Chemistry Channel
5:31and anti bonding molecular orbitals are empty. So as all the electrons in hydrogen molecule are paired up, it ...8 Jun 2020 · Uploaded by Edmerls

Use the mo diagram given to find the bond order for h2-.
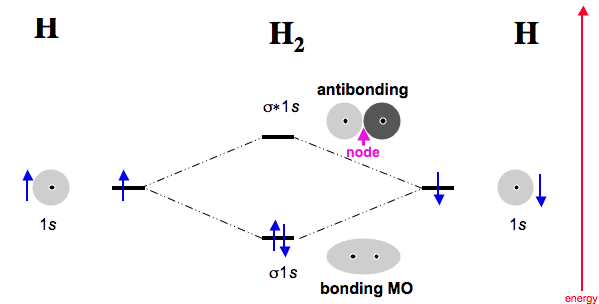
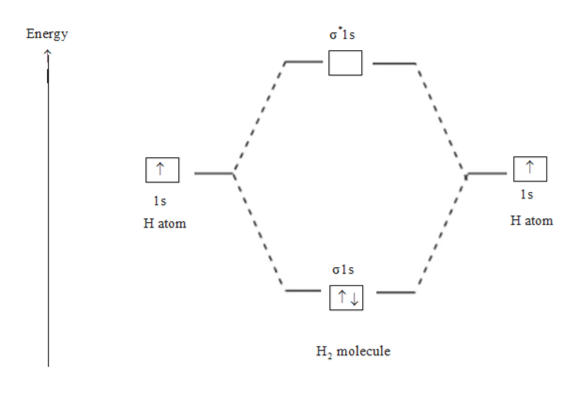
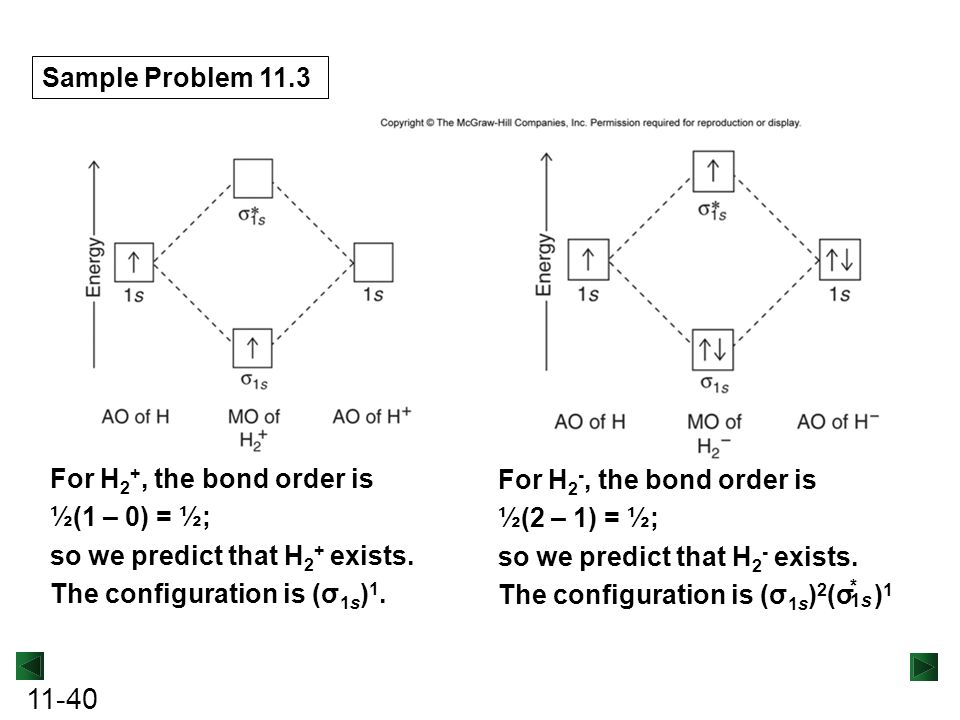
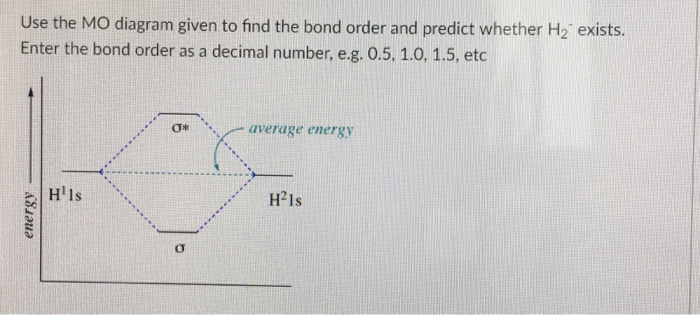
Use the MO diagram given to find the bond order and predict whether H2 exists. Enter the bond order as a decimal number, eg. 0.5. 1.0, 1.5, etc ?* ???Y-average energy H2ls ; Question: Use the MO diagram given to find the bond order and predict whether H2 exists. Enter the bond order as a decimal number, eg. 0.5. 1.0, 1.5, etc ?* ???Y-average ... Answer (1 of 2): In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. According to MOT number of atomic orbitals combined is equal to total number of molecular orbitals formed.Electronic configuration of H is 1s1. when two hydrogen atoms come closer, then on combi... A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ...
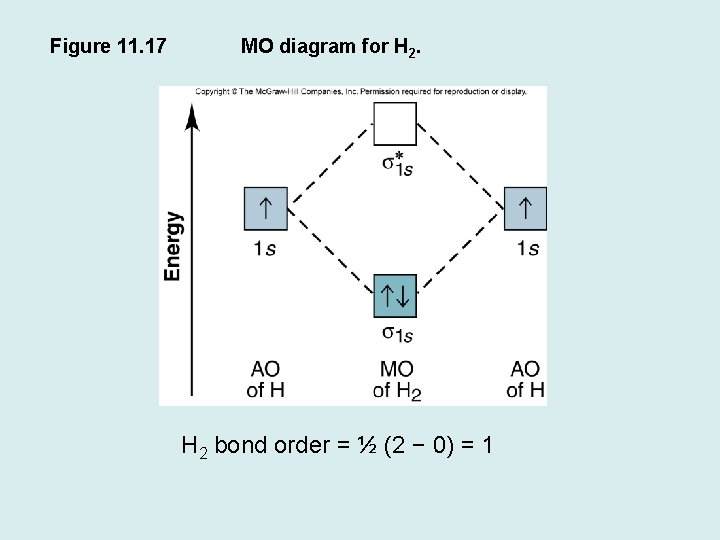
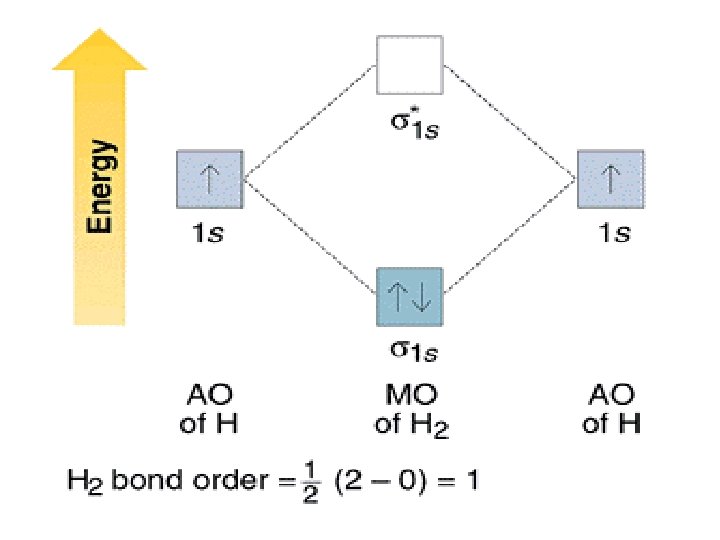
Use the mo diagram given to find the bond order for h2-.. 4:32The bond order of H2- is also calculated and the meaning of this ... and how to determine this for any ...3 Jan 2021 · Uploaded by Principia 3 Feb 2021 — For H2, bond order = 1/2 (2-0) = 1, which means H2has only one bond. The antibonding orbital is empty. Thus, H2 is a stable molecule. Again, in ... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine N b = 2 , Na =0. Bond order = 1. Positive value of bond order indicates that H 2 molecule is stable.. Bond order value of 1 means that two hydrogen atoms are connected by a single bond.. Greater value of bond order for H 2 molecule than H 2 + ion shows that two H 2 molecule is more stable than H 2 +.. Bond length of H 2 is smaller than that of H 2 + ion.. As no unpaired electron is present ...
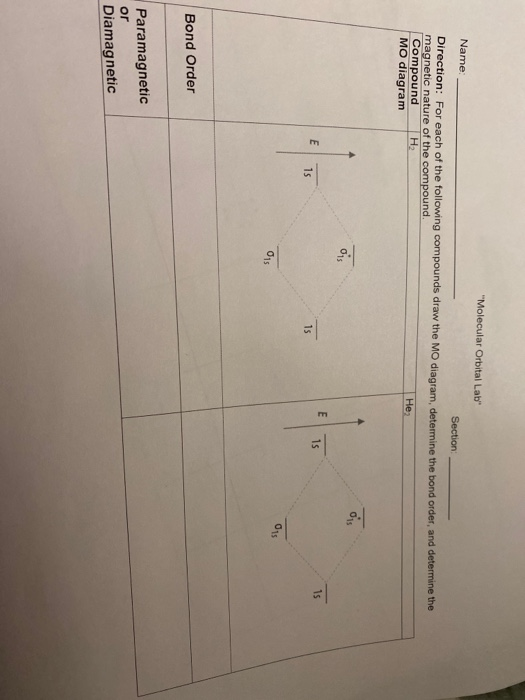
20, 7. (2 pts ea 8 pts) Consider the MO diagram shown on the right: 1 a) What is the bond order in N2: . 2p 2p b) Does adding an electron to N2 1 the bond order? or decrease increase 1 2a, c) How many anti-bonding orbitals are shown? 1a, 3: 1: 2: 2s 2s d) Assume the a-bond is aligned with the x-axis. 9:53In this video, we take a detailed look at the molecular orbitals of ... in this video are: bonding MO's ...6 Aug 2011 · Uploaded by Ben's Chem Videos Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Recall that the formula for bond order ...1 answer · Top answer: 0.5 2:20Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding ...9 Jun 2017 · Uploaded by chemistNATE
Fill in the molecular orbitals in the molecular orbital diagram for CO. One 2 s and three 2 p orbitals from carbon and one 2 s and three 2 p orbitals combine to form eight molecular orbitals in C O. The molecular orbitals in order of increasing energy are one sigma 2 s, one sigma 2 s star, two pi 2 p, one sigma 2 p, two pi 2 p star, and one ... Problem Details. Use an MO diagram to find the bond order and predict whether H 2− exists. Learn this topic by watching MO Theory: Bond Order Concept Videos. All Chemistry Practice Problems MO Theory: Bond Order Practice Problems. Q. Draw Lewis structures and MO diagrams for CN+, CN, and CN-. According to the Lewis model, which species is ... Each hydrogen atom contributes one electron, and thus, H− 2 has three electrons while H+ 2 has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one σ1s and one σ* 1s MO by conservation of orbitals. If you calculate their bond order, you get: BOH+ 2 = 1 2(Bonding − ... A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ...
Answer (1 of 2): In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. According to MOT number of atomic orbitals combined is equal to total number of molecular orbitals formed.Electronic configuration of H is 1s1. when two hydrogen atoms come closer, then on combi...
Use the MO diagram given to find the bond order and predict whether H2 exists. Enter the bond order as a decimal number, eg. 0.5. 1.0, 1.5, etc ?* ???Y-average energy H2ls ; Question: Use the MO diagram given to find the bond order and predict whether H2 exists. Enter the bond order as a decimal number, eg. 0.5. 1.0, 1.5, etc ?* ???Y-average ...

Use The Molecular Orbital Energy Level Diagram To Show That N 2 Would Be Expected To Have A Triple Bond F 2 A Single Bond And Ne 2 No Bond
Draw Mo Diagram Of Co And Calculate Its Bond Order Sarthaks Econnect Largest Online Education Community

Complete This Molecular Orbital Diagram For Cn Then Determine The Bond Order Note That The 1s Homeworklib

Write Molecular Orbital Configuration Of C2 Predict Magnetic Behaviour And Calculate Its Bond Order H7ch14qq Chemistry Topperlearning Com
Give The Molecular Orbital Energy Diagram Of N2 And O2 Write The Bond Order Of N2 And O2 Sarthaks Econnect Largest Online Education Community

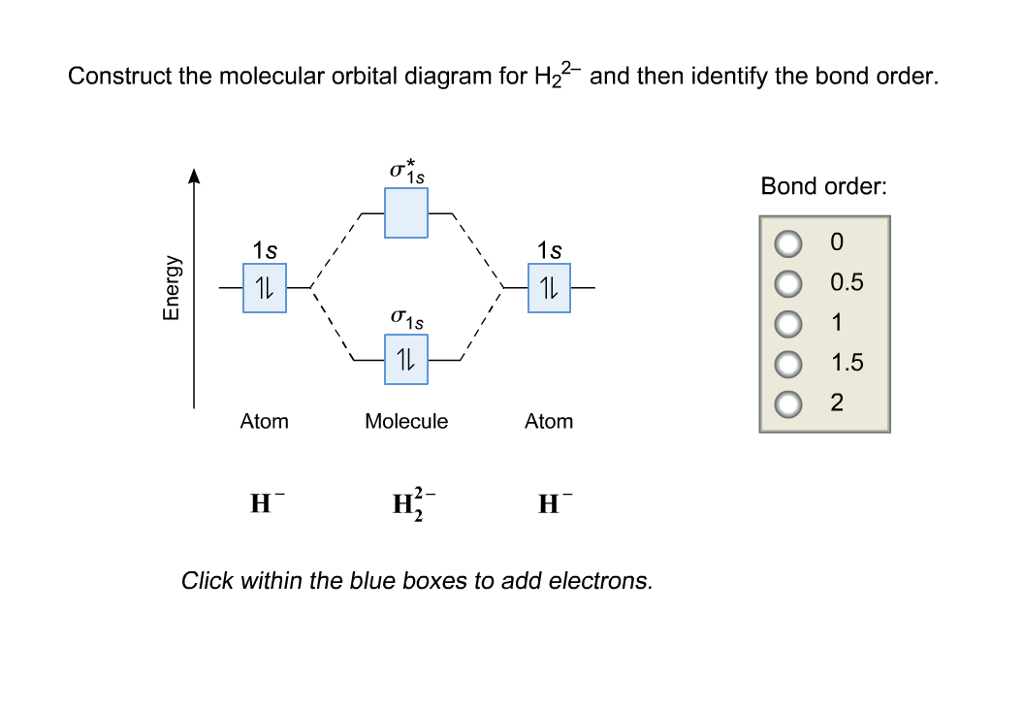
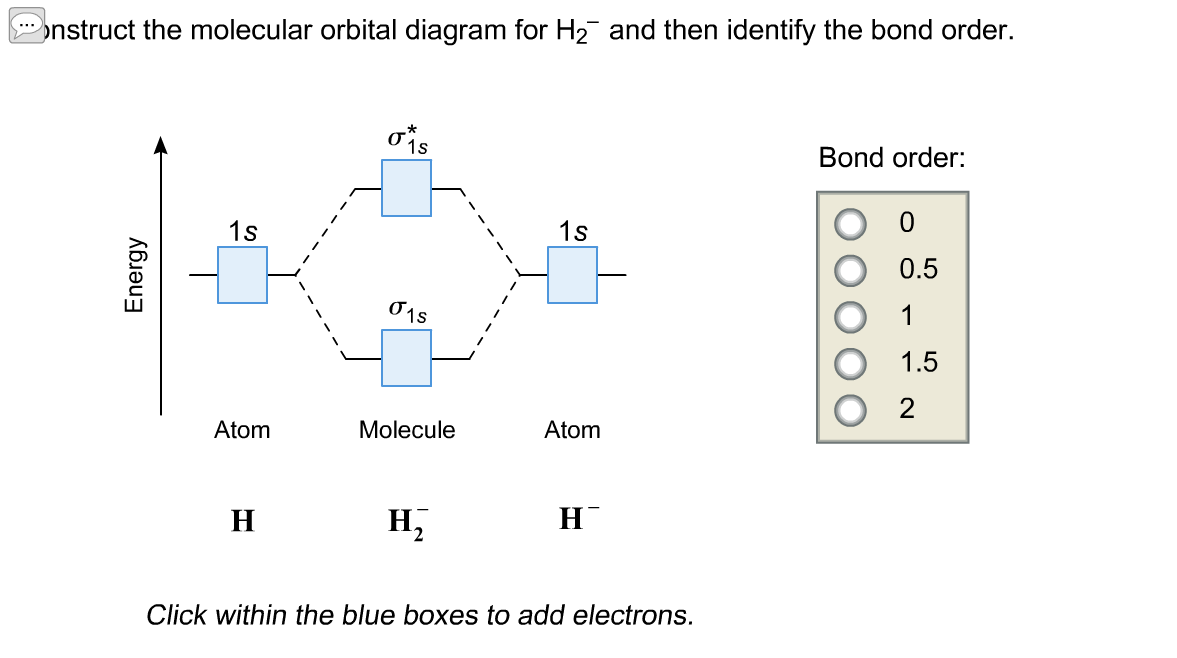
Construct The Molecular Orbital Diagram For H2 And Then Identify The Bond Order Bond Order Click Homeworklib







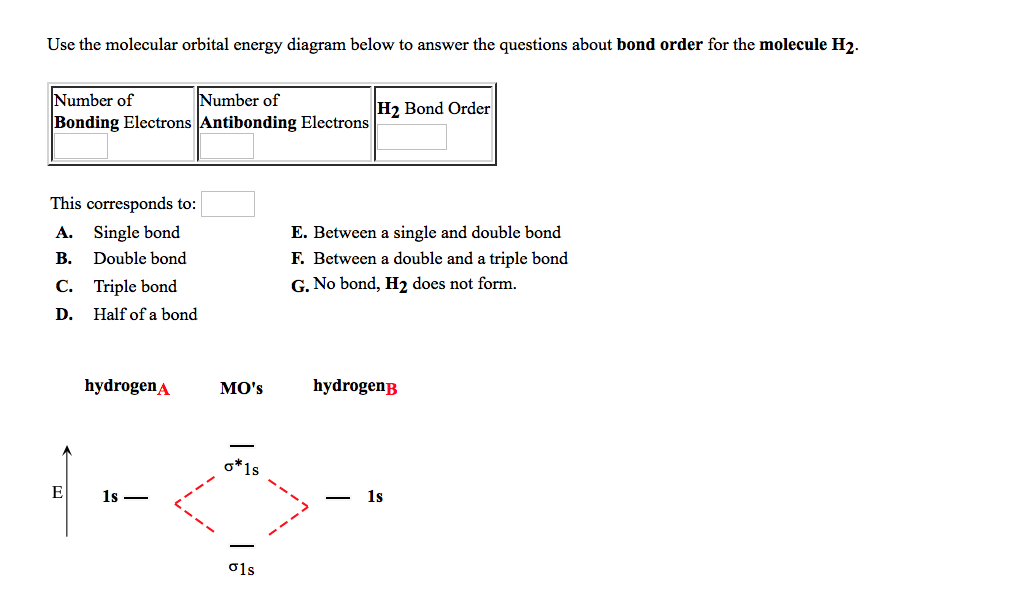




0 Response to "41 use the mo diagram given to find the bond order for h2-."
Post a Comment