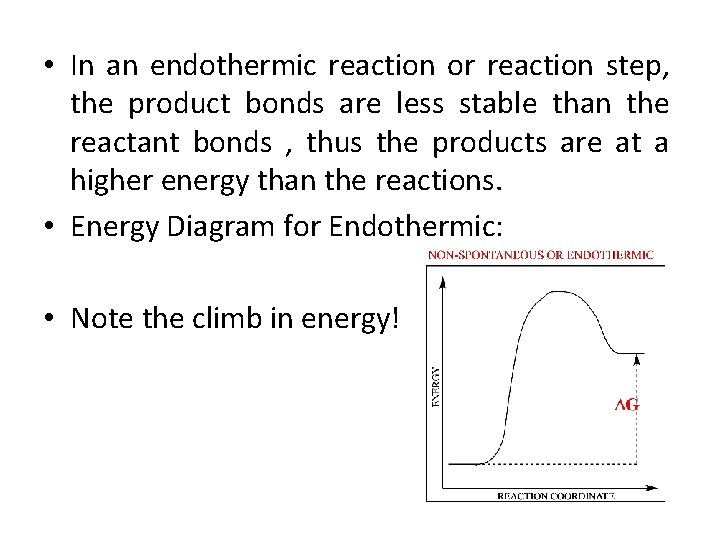
43 endothermic reaction energy diagram
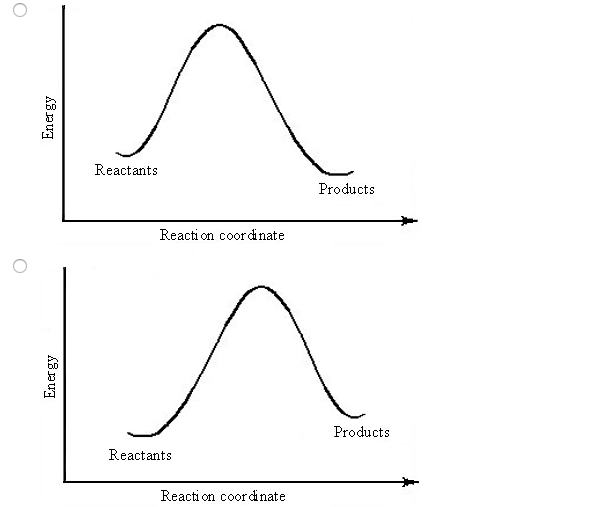
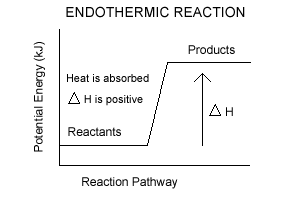
A reaction that takes in heat energy so the temperature goes down on a energy profile diagram is it a exothermic or endothermic reaction if the activation energy is small. In the case of an endothermic reaction the reactants are at a lower energy level compared to the productsas shown in the energy diagram below. In an endothermic reaction, the reactants absorb heat energy from the surroundings to form products. Thus, the products formed have more energy than the reactans, H products > H reactants. Therefore, ΔH is positive. Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show:
Draw an energy diagram for an endothermic reaction without a catalyst (use a solid line) and with a catalyst (use a dotted line). Label all parts of the diagram. Explain what a catalyst is and how a catalyst influences the rate of a reaction. Answer: A Catalyst lowers the activation energy so it increases the rate of reaction.

Endothermic reaction energy diagram
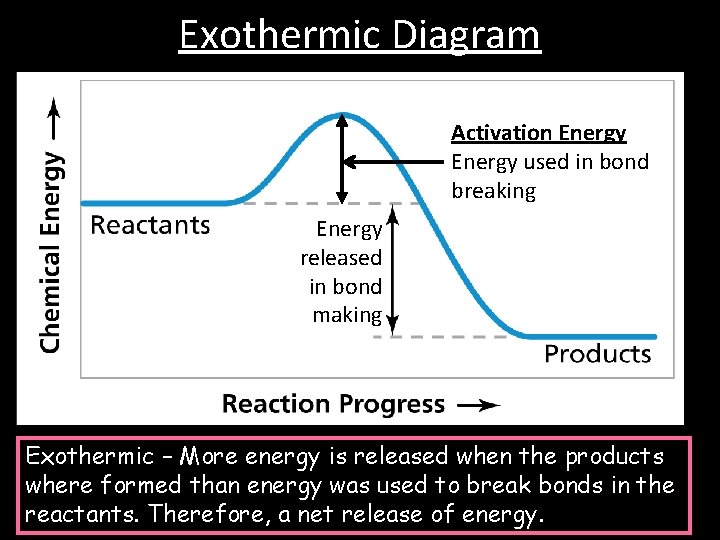
Exothermic reactions uses/loses energy/heat during the reaction, so when drawing the energy diagram, you want the reactants on the graph to be higher than the ... Based on the following energy diagram, is the | Chegg.com. 3. Based on the following energy diagram, is the reaction exothermic or endothermic? How many steps are in this reaction? How many transition states and intermediates? free energy, kJ/mol reaction coordinate 4. Based on the following energy diagram, is the reaction exothermic or ... Chemistry Q&A Library Sketch an energy diagram graph representing an endothermic reaction, and label the following. a. Average energy of reactants b. Average energy of products c. Activation energy d. Amount of energy absorbed during the reaction
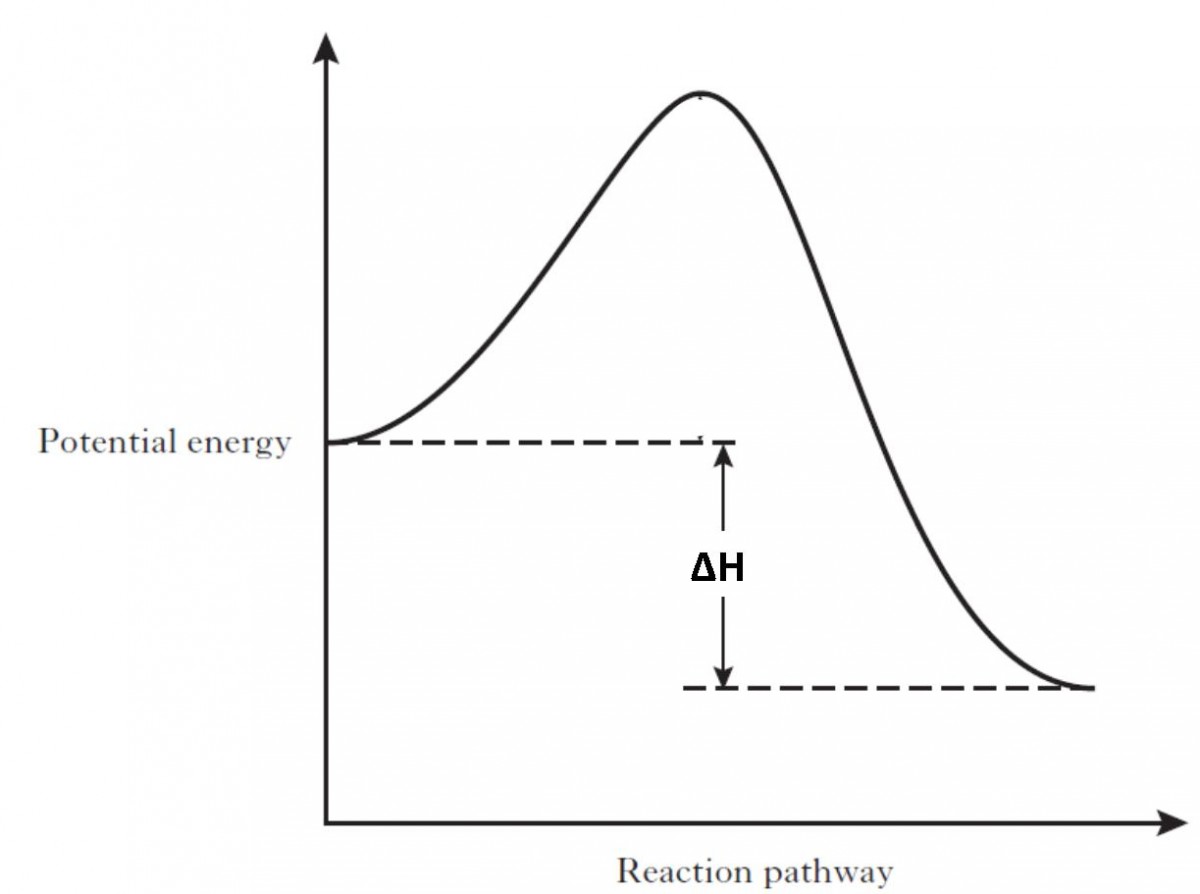
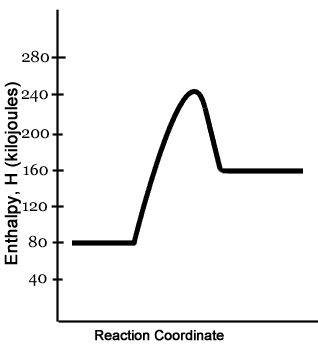
Endothermic reaction energy diagram. The energy level decreases in an exothermic reaction. This is because energy is given out to the surroundings. Graph of energy against progress of reaction. Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy. Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants. 9 Jul 2021 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
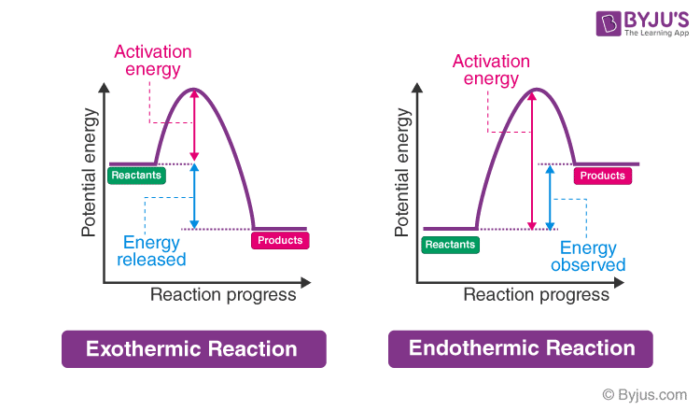
In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. Endothermic Reaction Energy Diagram — For example, plants take in energy from the sun and convert carbon dioxide and water into glucose and oxygen. An ...Enthalpy change of the reaction: Positive (ΔH>...Temperature of the surroundings: Decreases as ...Examples: Melting of ice, photosynthesis, evap...Definition: The system absorbs heat from surro... Describe the potential energy diagram for an exothermic reaction. Define activation energy. What was Sisyphus's punishment? Sisyphus was ordered to repeatedly ... Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is ...
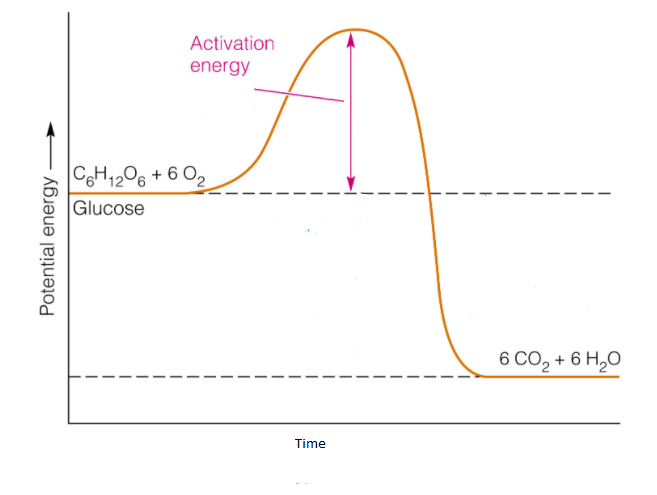
Energy changes accompany chemical reactions. Energy diagrams are quite useful in illustrating these changes on a continuous basis as the reaction proceeds. Terms such as "activation energy" (E a), "transition state" (*), and "enthalpy change" are easy to define by referring to a graph such as Figure 1. Endothermic and exothermic reactions are ... ENERGY IS A REACTANT, SO THE REACTION IS ENDOTHERMIC AND ΔH IS POSITIVE! Page 5. EQUATIONS &. ENERGY DIAGRAMS. • WE CAN USE AN ENERGY DIAGRAM ...11 pages Endothermic Reaction Energy Diagram. bbc gcse bitesize energy level diagrams energy level diagrams during a chemical reaction energy is either taken in or given out during exothermic reactions energy is given out this means that the energy of the products will be lower than the energy of the reactants this can be represented using an energy level diagram endothermic vs exothermic reactions ... Chemistry Q&A Library Sketch an energy diagram graph representing an endothermic reaction, and label the following. a. Average energy of reactants b. Average energy of products c. Activation energy d. Amount of energy absorbed during the reaction
Based on the following energy diagram, is the | Chegg.com. 3. Based on the following energy diagram, is the reaction exothermic or endothermic? How many steps are in this reaction? How many transition states and intermediates? free energy, kJ/mol reaction coordinate 4. Based on the following energy diagram, is the reaction exothermic or ...
Exothermic reactions uses/loses energy/heat during the reaction, so when drawing the energy diagram, you want the reactants on the graph to be higher than the ...

Question Video Identifying Steps In A Reaction Profile Diagram For A Two Step Chemical Reaction Nagwa
2 2b Reaction Rates Chemical Equilibrium Interpret Diagram Note The Videos In The Helpful Videos Section Should Be Viewed Interpret Diagram Showing Activation Energy Along A Reaction Pathway 2 2b Activation Energy Ea The Minimum Energy
The Following Diagram Shows The Energy Of A Reaction As The Reaction Progresses Label Each Of Homeworklib
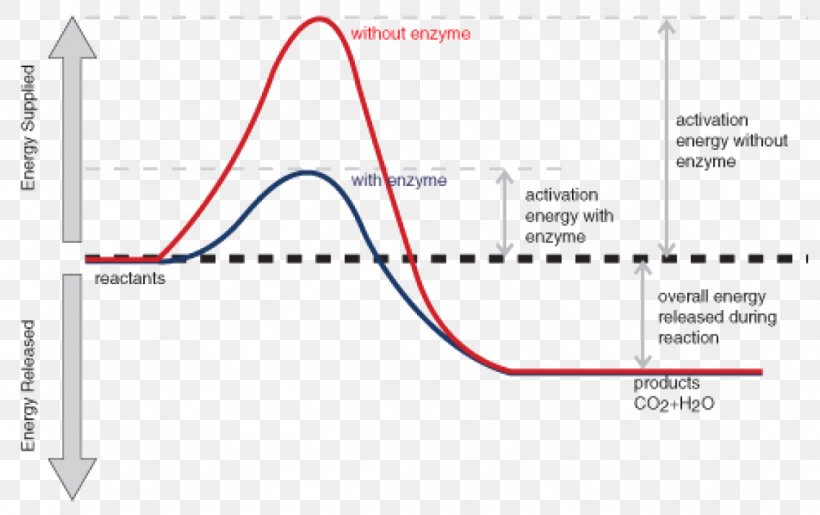
Activation Energy Chemical Reaction Enzyme Exothermic Reaction Png 1111x698px Activation Energy Activation Adenosine Triphosphate Anabolism Area
Igcse Chemistry 4 14 Represent Exothermic And Endothermic Reactions On A Simple Energy Level Diagram
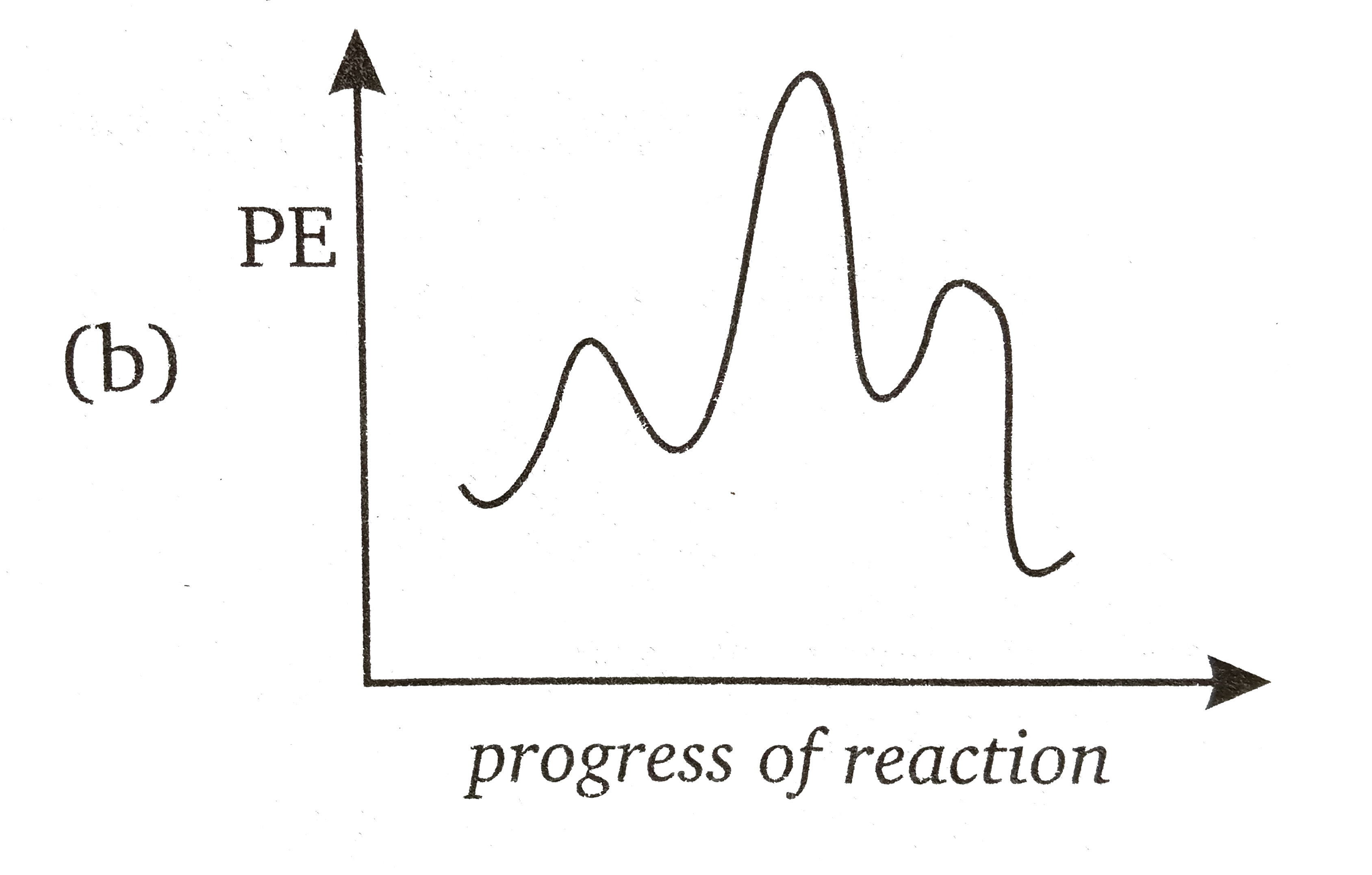
Draw An Energy Profile Diagram For A Three Step Reaction In Which First Step Is Slowest And Last Step Is Fastest Assume That Reaction Is Exothermic
An Endothermic Reaction With High Activation Energy For The Forward Reaction Is Given By The Diagram Sarthaks Econnect Largest Online Education Community

For An Endothermic Reaction Energy Of Activation Is Ea And Enthalpy Of Reaction Is Dh Both Of These In Kj Mol Minimum Value Of Ea Will Be From Chemistry Chemical Kinetics Class 12


















0 Response to "43 endothermic reaction energy diagram"
Post a Comment