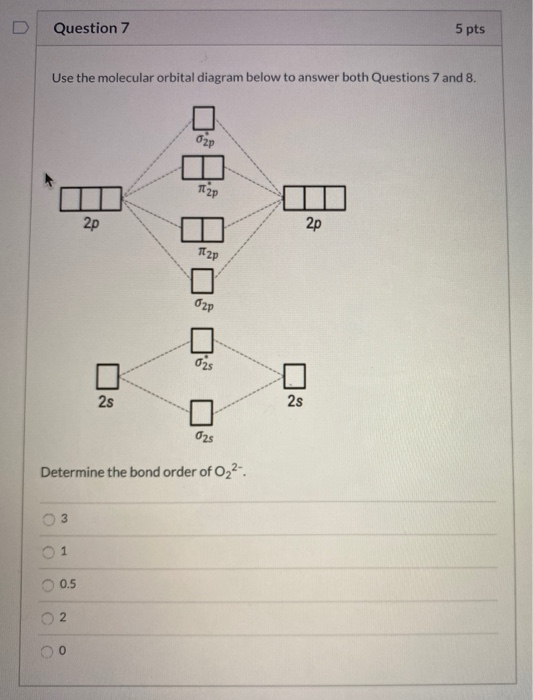
43 molecular orbital diagram for o2 2
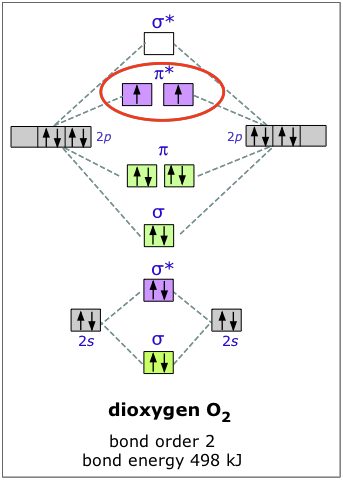
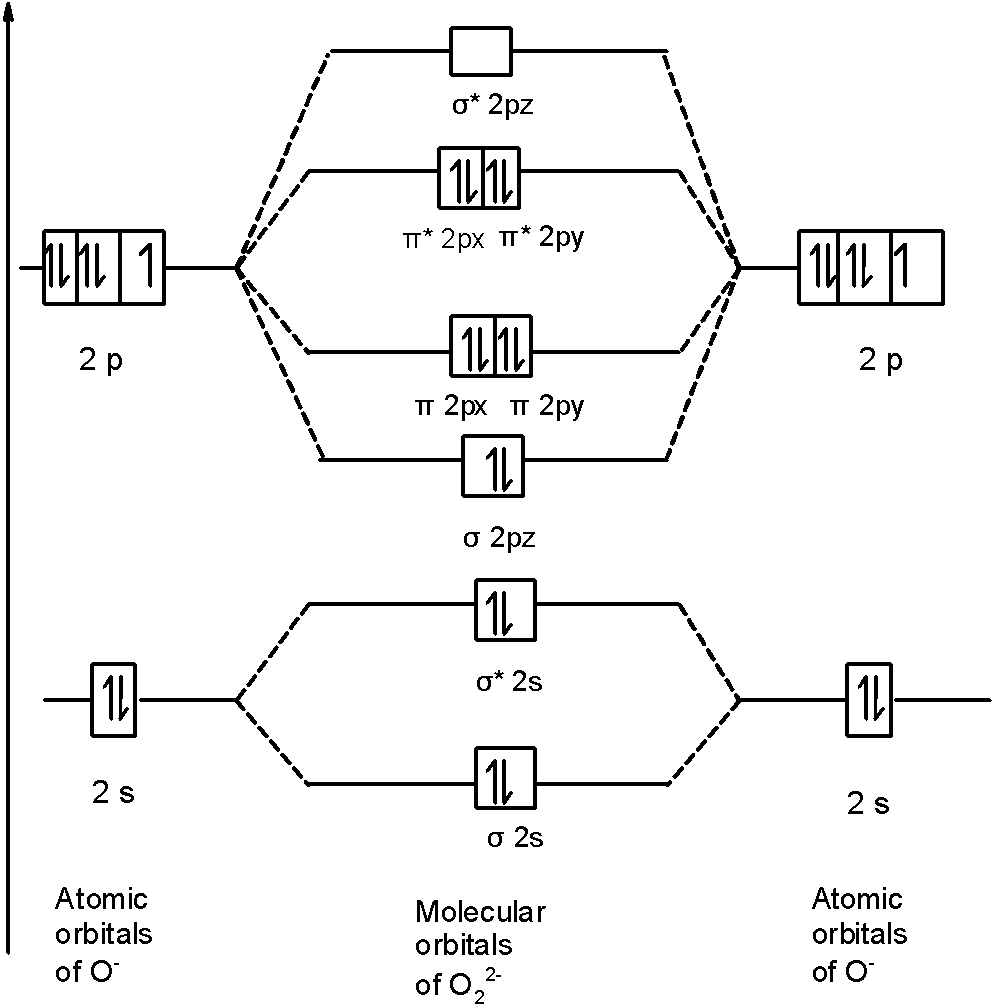
From that diagram, you can then easily fill out what the O2- and O2+ MO diagrams should be—and that is in the second photo I included. The first photo is straight from a 2006 edition Pearson general chemistry textbook, and it shows you what the molecular orbital (MO) diagram for O2 is. S(0) and S 2 O 3 2− produced as intermediates [37,38], with the relative completion depending on the pH or the microbial community. In some species, for example, pH affects the relative rates of different steps of S 2 O 3 2− oxidation via the sox pathway...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
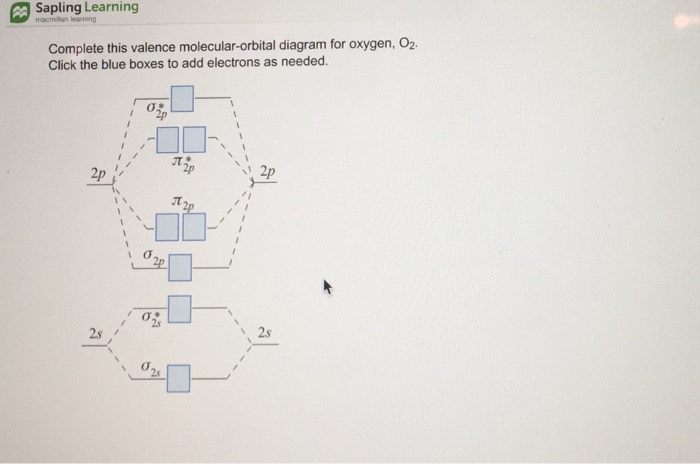
Molecular orbital diagram for o2 2
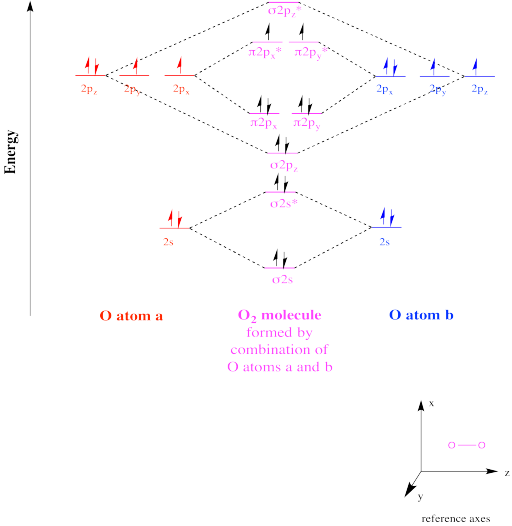
Molecular Orbital theory starts by assuming that the three atomic p orbitals on the O atoms overlap to form three molecular π orbitals that extend over the whole molecule. We end up with two electrons in a bonding π orbital; two electrons in a nonbonding #π^n# orbital; and no electrons in an antibonding... The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions If we arbitrarily define the Z axis of the coordinate system for the O2 molecule as the axis along which the bond forms, the 2pz orbitals on the adjacent... Start studying Molecular Orbital Theory. Learn vocabulary, terms and more with flashcards, games and other study tools. dia, para (O2 has 2 unpaired electrons in its M.O., N2 has none). When drawing heteronuclear diatomic molecular orbital diagrams; remember that the...
Molecular orbital diagram for o2 2. Molecular Orbital Energy Diagrams. Bond Order. Bonding in Diatomic Molecules. Experiments show that each O2 molecule has two unpaired electrons. The Lewis-structure model does not predict the presence of these two unpaired electrons. A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row - MO diagrams for Inorganic complexes. • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory. **Physical Chemistry** **Thermodynamics, Structure, and Change 10th Edition Solutions Peter Atkins, Julio de Paula** **ISBN-13: 9781429290197** Download the Solutions manual for this textbook **Order it via email: markrainsun"@"gmail(.)com** ​ **Table of Contents** **Foundations** A Matter B Energy C Waves **Part 1 Thermodynamics** **1. The properties of gases** Topic 1A The perfect gas Topic 1B The kinetic model Topic 1C Real gases **Impact** …O...
Molecular orbital (MO) theory defines the building of molecular orbital diagram on the basis of complying with main points . Also Watch Molecular orbital diagram of O2 , O2+2 , 02 -2 ( in Urdu / Hindi). Simplest Trick to Calculate Bond Order c. a violation of the octet rule. d . the molecular orbital diagram for O 2. e . hybridization of atomic orbitals in O 2. 29 . Which of the following electron distributions among the molecular orbitals best describes the NO molecule ? σ 2s σ 2s * π 2 py = π 2 px σ 2 pz π 2 py * = π 2 px * σ 2 pz *. Hello guys ! I have my finals in less than a month and I'm definitely stuck on this part of the program; I would like someone trying to explain me how you actually make a molecular orbital diagram through a relatively simple example like H2 or less simple like O2 in the form of an infographic, with a lot of arrows in it or something in this kind :) This would really help me, thanks in advance for your time ! Have a wonderful day ! Molecular Orbital Energy Diagrams. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 8). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right.
In o 2 and f 2 there is a crossover of the sigma and the pi ortbials. Information from the mo diagram justify o2s stability and show that i... Bonding order is 2 and it is paramagnetic. The two hydrogen 1s orbitals are premixed to form a 1 σ and b 2 σ mo. File:Oxygen molecule orbitals diagram.JPG. From Wikimedia Commons, the free media repository. Jump to navigation Jump to search. DescriptionOxygen molecule orbitals diagram.JPG. English: Molecular orbital energy diagram for O2. Date. 19 March 2008, 03:01:58. Bonding order is 2 and it is paramagnetic. Molecular orbital theory describes the distribution of electrons in molecules in much the same w...
Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
Learn about the molecular orbital diagram for o2 using these free and printable molecular orbital diagram as your reference in understandin...
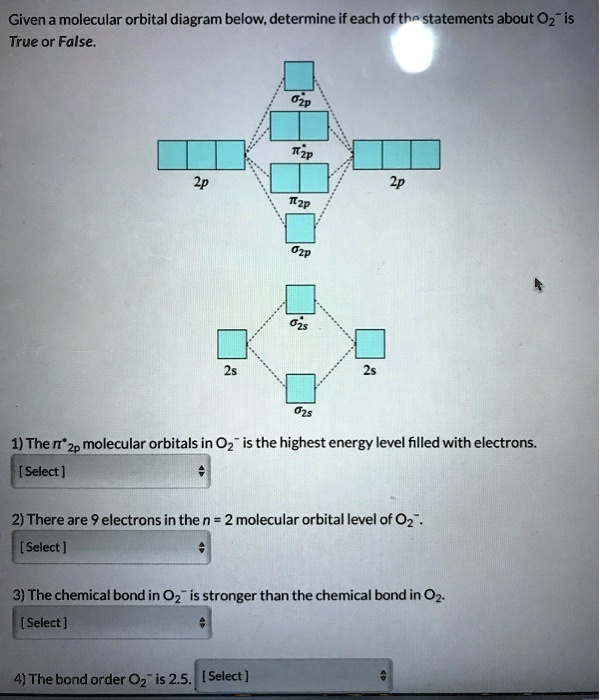
Solved Given A Molecular Orbital Diagram Below Determine If Each Of The Statements About 02 Is True Or False 1 The It 2p Molecular Orbitals In 02 Is The Highest Energy Level Filled With
The molecular orbital diagram representing this order of energy levels is shown in fig. This kind of mixing of orbitals or symmetry interaction is not applicable for O2 and F2 molecule formation because of larger energy gap between 2s and 2p orbitals for these atoms.
Molecular Orbital Theory. I'm having a lot of trouble with this stuff. I don't really know how to start these questions (such as how to draw a correlation diagram) Well, this stuff is pretty hard to explain in text alone but I'll give it a go. Feel free to ask clarification questions. I'll use your example of O2
Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals.
O2 Molecular Orbital Diagrams U2013 101 Diagrams. Frontier Molecular Orbitals And Electronic Terms Of 2 The. O2 Molecular Orbital Diagrams U2013 101 Diagrams. Grafik Molecular Orbital Diagram For Of2 Hd Version. What Is The Bond Angle Of Of2.
Remember: When two oxygen atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals. They are flipped...
What is the highest occupied molecular orbital (HOMO) in each one? Assume the molecular orbital diagram in Figure 9.16 applies to all of them. (a) Assuming the electronic structure of the molecule can be described using the molecular orbital energy level diagram in Figure 9.16 , answer the...
Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals.
Hello, So I am currently reading up about bonding and anti-bonding orbitals in Molecular Orbital Theory and am very confused as to how they relate to the actual bonds. I see you can use the Bond Order Formula to determine HOW MANY bonds the compound will have, but I don't see how the different orbitals actually relate to the bonds a compound makes, like what orbitals are doing what and which bond is formed by which orbital or whatever. For example, I am looking at a MO diagram of O2 and I get ...
Information from the mo diagram justify o2s stability and show that its bonding order is 2. Printable o2 molecular orbital diagrams are ava...
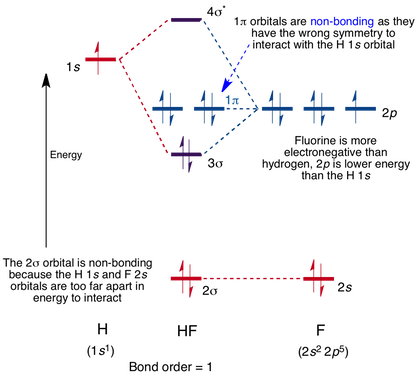
I was thinking about doing a molecular orbital diagram, but I was intimidated by the rules for heteronuclear MO diagrams, and when I thought about an MO diagram for a continuous, indefinitely extending amorphous solid like SiO2 (or a hypothetical "silica-like" version of CO2, to compare its diagram to that of simple three-atom CO2), I realized I had no idea what I was going to do. Is there a comprehensible explanation of why CO2 is most stable in its double-bonded small molecule form, while SiO...
draw the molecular orbital diagram of O2 or N2 - Brainly.in (Ola Carlson). The orbital diagram for a diatomic molecule is. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory.
Can you draw good dot structures that correspond to each of these ions or molecules. Molecular orbital diagram of o 2 electronic configurat...

How To Draw Molecular Orbital Diagram Of O2 Simplest Trick Chemistry Best Online Free Chemistry Class 9 12
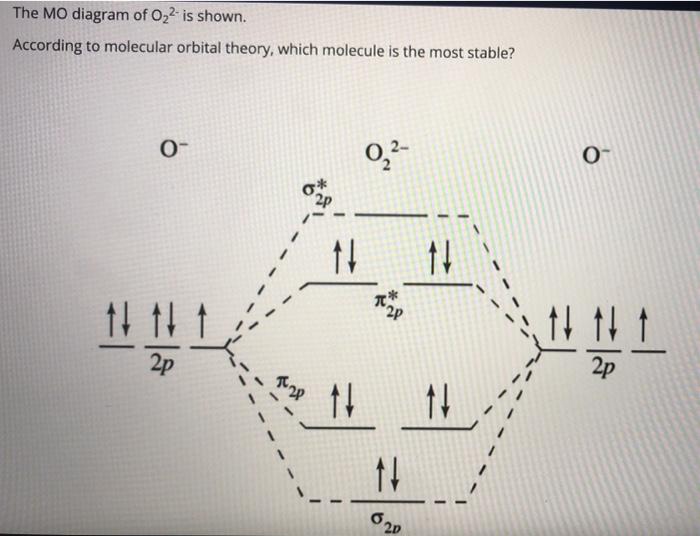
The bond length in the oxygen species o2 o2 o2 o22 can be explained by the positions of the electrons in molecular orbital theory.
Draw The M O Diagram For Oxygen Molecule And Calculate Its Bond Order And Show That O2 Is Paramagnetic Sarthaks Econnect Largest Online Education Community
Start studying Molecular Orbital Theory. Learn vocabulary, terms and more with flashcards, games and other study tools. dia, para (O2 has 2 unpaired electrons in its M.O., N2 has none). When drawing heteronuclear diatomic molecular orbital diagrams; remember that the...

Draw The Molecular Orbital Energy Diagram For Oxygen Molecule O2 And Show That I It Has A Double Bond Ii It Has Paramagnetic Character From Chemistry Chemical Bonding And Molecular Structure Class 11 Cbse
The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions If we arbitrarily define the Z axis of the coordinate system for the O2 molecule as the axis along which the bond forms, the 2pz orbitals on the adjacent...
Molecular Orbital theory starts by assuming that the three atomic p orbitals on the O atoms overlap to form three molecular π orbitals that extend over the whole molecule. We end up with two electrons in a bonding π orbital; two electrons in a nonbonding #π^n# orbital; and no electrons in an antibonding...

Molecular Orbital Theory Homodiatomics Use The Molecular Orbital Model To Fully Describe The Bonding In O2 O2 O2 And O22 Determine Which Of The Following Statements Are True And Which Are

Draw Molecular Orbital Energy Diagram Of O2 Molecule And Mention Its Bond Order Explain Why O2 Brainly In

Pleaseshow Me The Energy Level Diagrams Of O2 O2 O2 2 Chemistry Chemical Bonding And Molecular Structure 7027301 Meritnation Com

Consider The Molecular Orbital Diagram For The Ion O 2 2 Predict The Bond Order A 3 0 B 2 5 C 1 0 D 2 0 E 1 5 Consider The Following Statements Will The Ion Be Paramagnetic Or Study Com







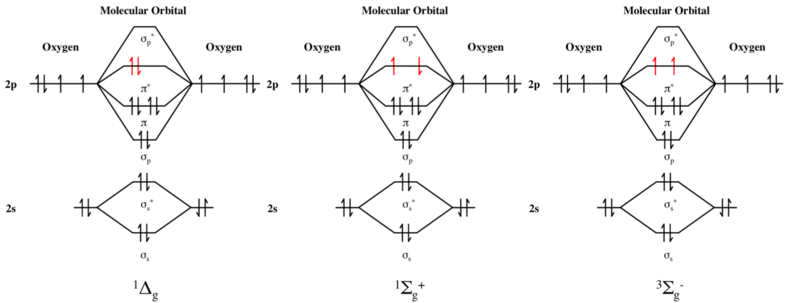















0 Response to "43 molecular orbital diagram for o2 2"
Post a Comment