45 mo diagram for he2
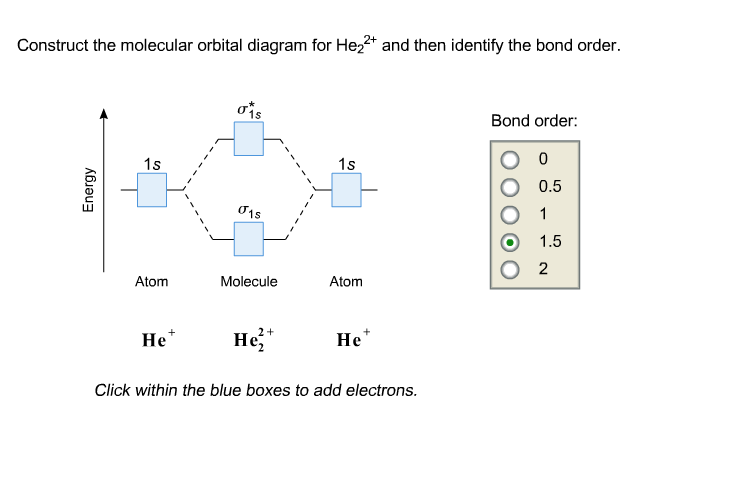
Complete step by step answer: The molecular orbital theory assumes that the atomic orbitals in molecules lose their identity and the electrons in these ... Mo Diagram He2. Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. According to Molecular Orbital (MO) Theory, two atoms mix their orbitals to form one that is spread out over both atoms. The mixing of two.
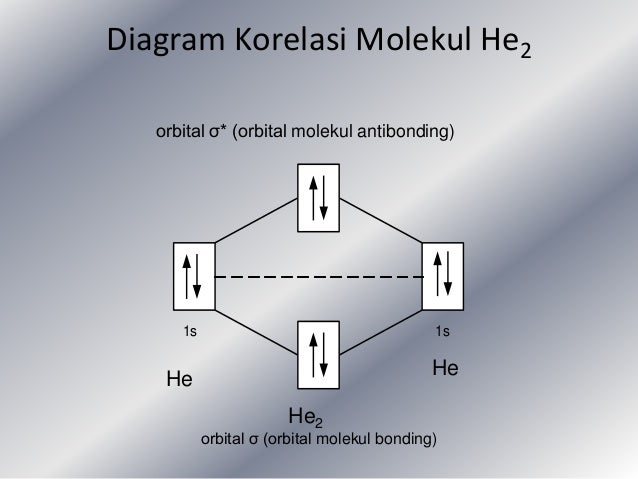
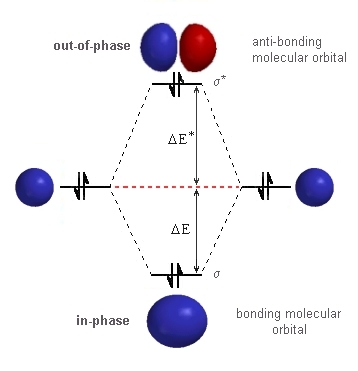
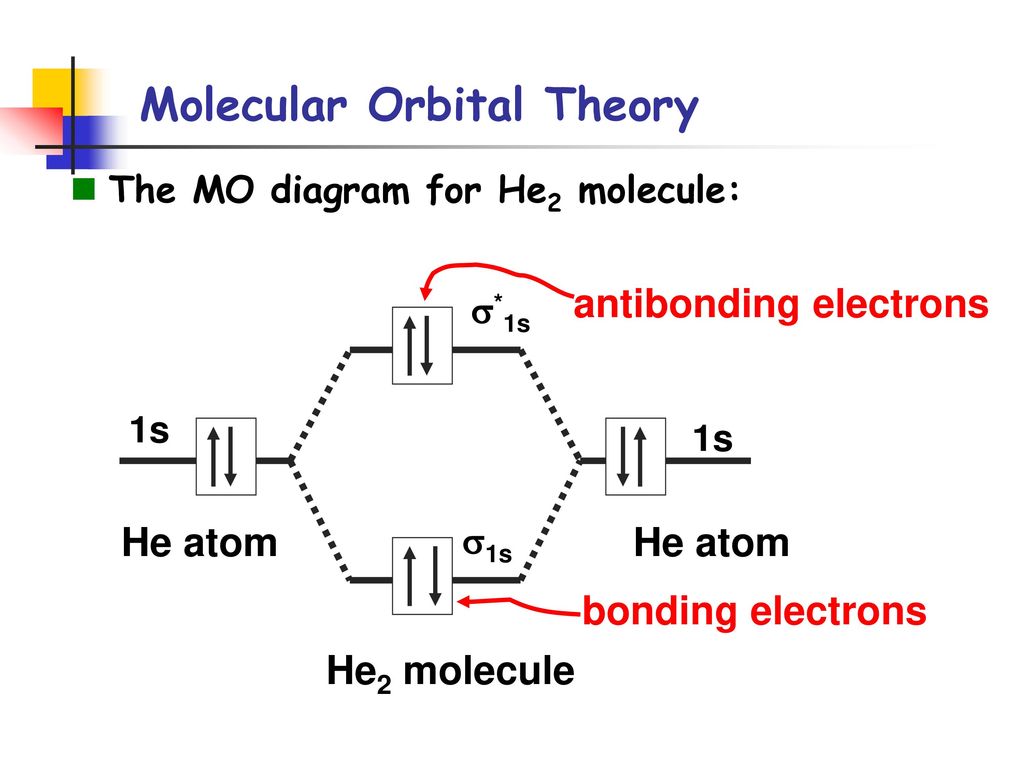
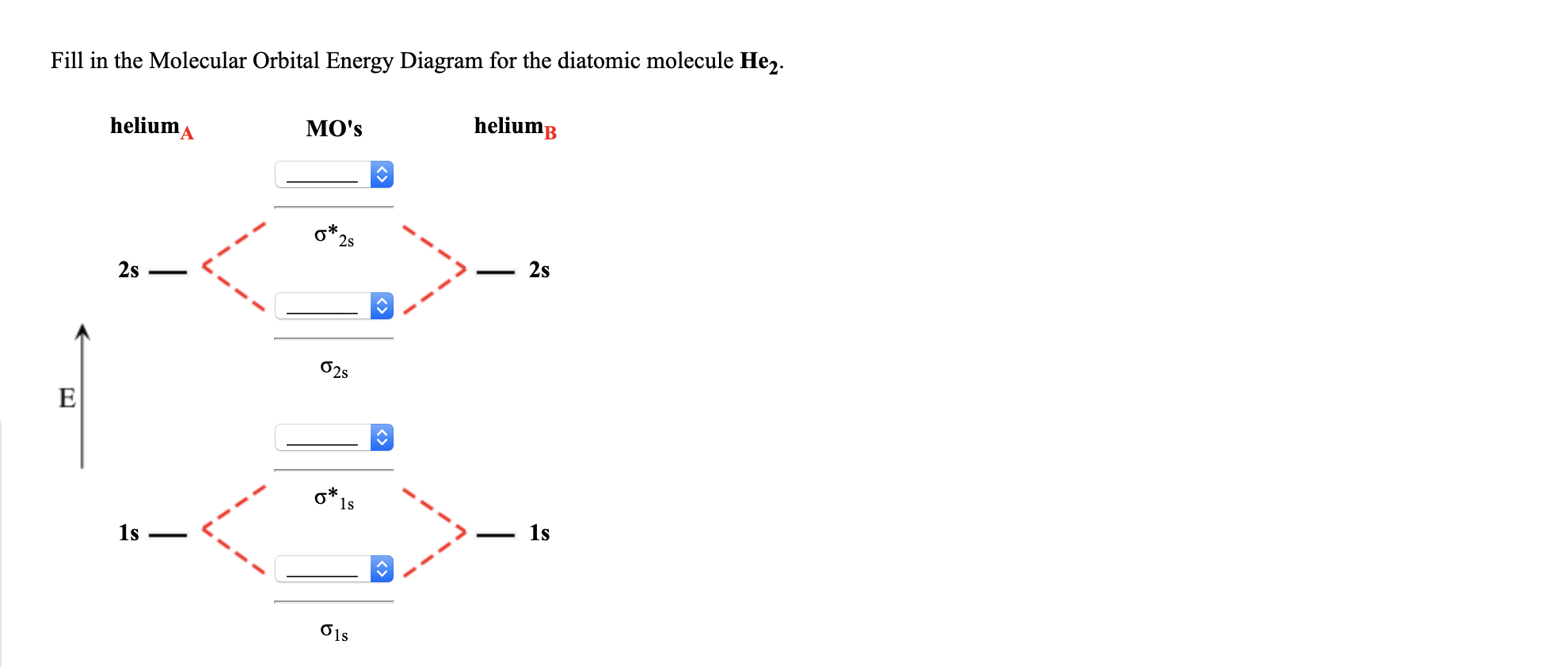
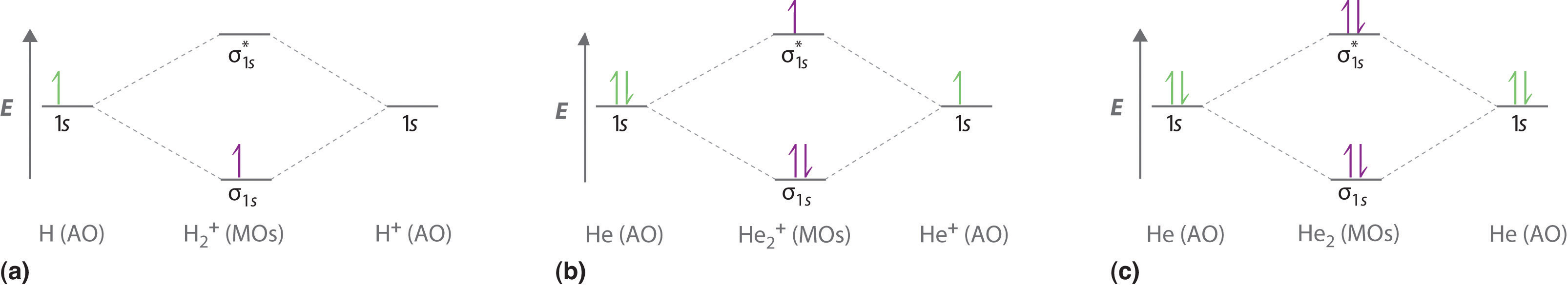
In He2 (dihelium), the two 1s atomic orbitals overlap to create two molecular orbitals: sigma(1s) and sigma(1s)*. You fill these molecular orbitals with the...
Mo diagram for he2
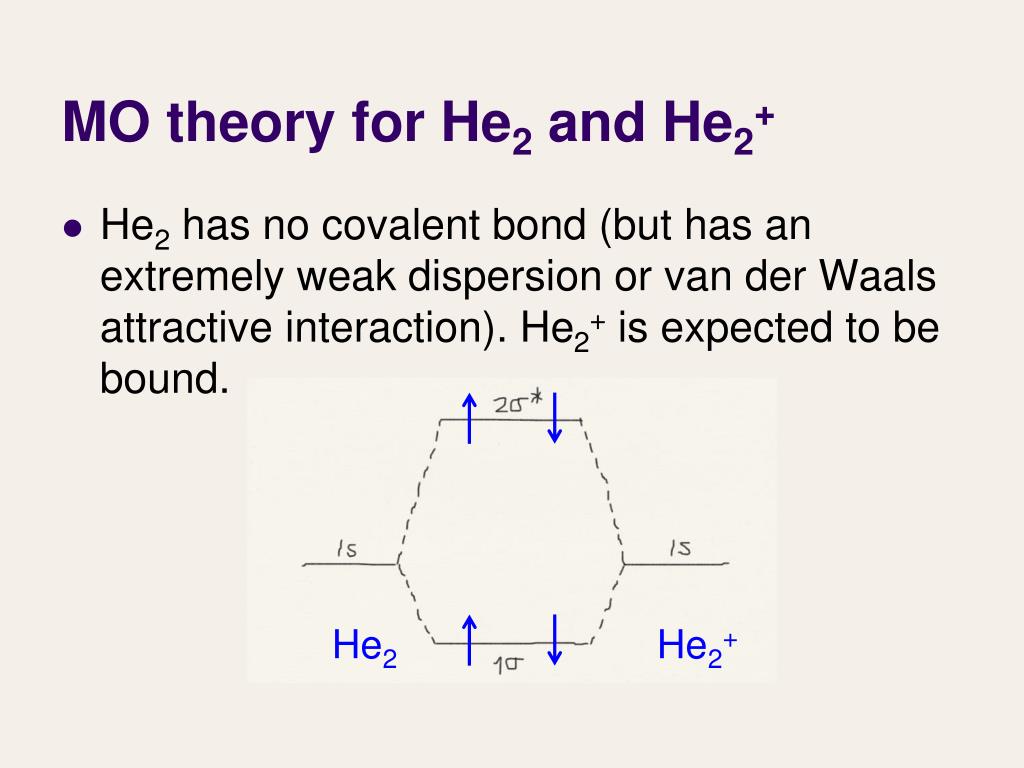
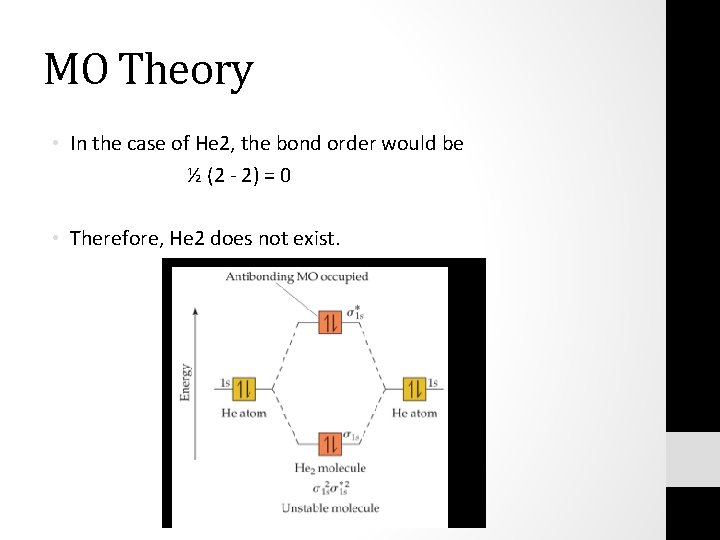
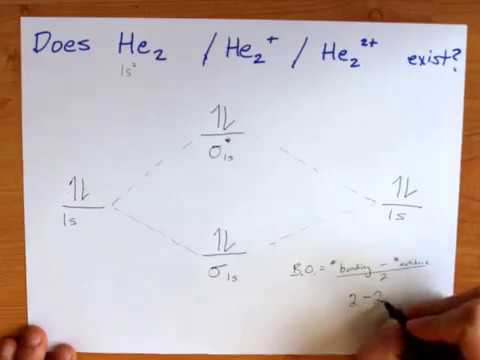
Molecular orbital diagram has been drawn for the given molecule. This has totally 4 electrons in it. In molecular orbital diagram, it is clearly shown that the bonding orbital and the antibonding orbitals has two electrons each. Therefore, the number of bonding electrons are 2 and the number of anti-bonding electrons are 2. A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ... 1 answerIn the molecular di-cation of helium ion, two electrons are less than helium atom. Two electrons are to be filled in the molecular orbitals.
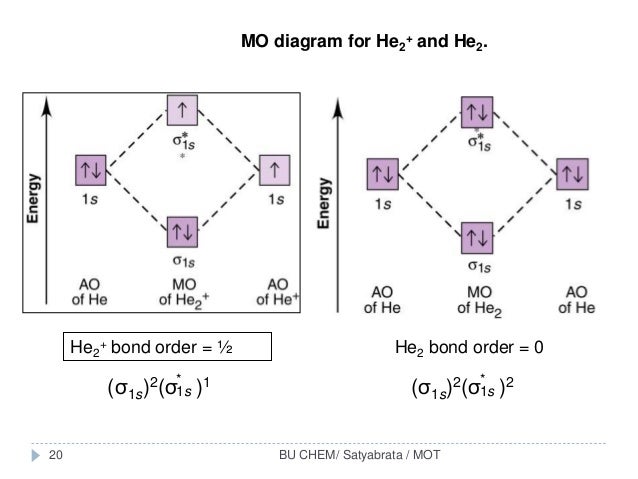
Mo diagram for he2. FREE Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click...1 answer · Top answer: Concepts and reason Bond order is the number, which indicates the total number of bonds present between two atoms. Bond order describes the bond strength ... He2+ MO diagram. Eg: Li + H; Li has 1s + 2s, while H has 1s. This mix to form a sigma orbital from H1s+Li2s, a sigma* orbital and H1s-Li2s. The bond order of a simple molecule can be determined by looking at the number of electrons in bonding and antibonding molecular orbitals. Molecular Orbital Diagram For He2 2+ Two electrons total, both occupy the sigma orbital, two more electrons in bonding than antibonding He2 is not possible. Please note the diagram is for He2+ but the He-H is very similar answered Mar 21 '13 at A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and ... How to write simple Molecular Orbital Diagrams and determine the Bond order
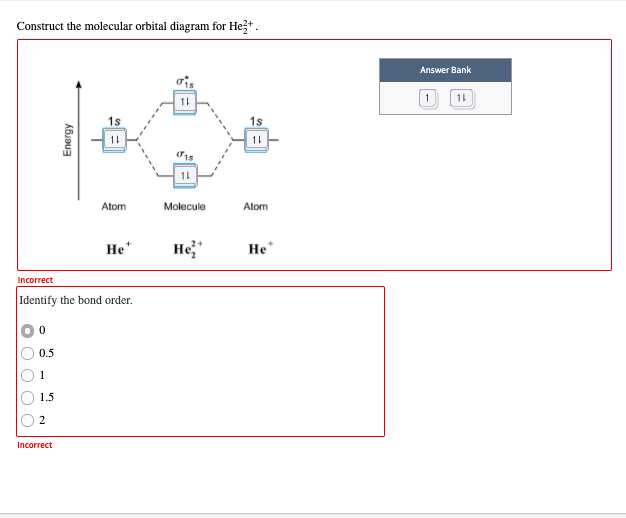
Construct the molecular orbital diagram for he 2 and then identify the bond order. Click calculate to proceed. The lewis structure for h2 is h h predicting a single bond between each hydrogen atom with two electrons in the bond. Please note the diagram is for he2 but the he h is very similar eg. He h forms a very weak bond. Transcribed image text: Draw the molecular orbital diagram for Hez Drag the appropriate labels to their respective targets. Reset th Atomic orbital Molecular orbitals Atomic orbital 11 ॥ Antibonding ls ls Energy # He atom Bonding Het ion 1+1 11 He2+ ion. Previous question. A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 . 1 answerIn the molecular di-cation of helium ion, two electrons are less than helium atom. Two electrons are to be filled in the molecular orbitals.
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ... Molecular orbital diagram has been drawn for the given molecule. This has totally 4 electrons in it. In molecular orbital diagram, it is clearly shown that the bonding orbital and the antibonding orbitals has two electrons each. Therefore, the number of bonding electrons are 2 and the number of anti-bonding electrons are 2.

Oneclass Construct The Molecular Orbital Diagram For He2 2 And Sapling Learning Map D Mcanoe Constru

Construct The Molecular Orbital Diagram For He2 And Then Identify The Bondorder Bond Order Click Within The Blue Boxes To Add Electrons

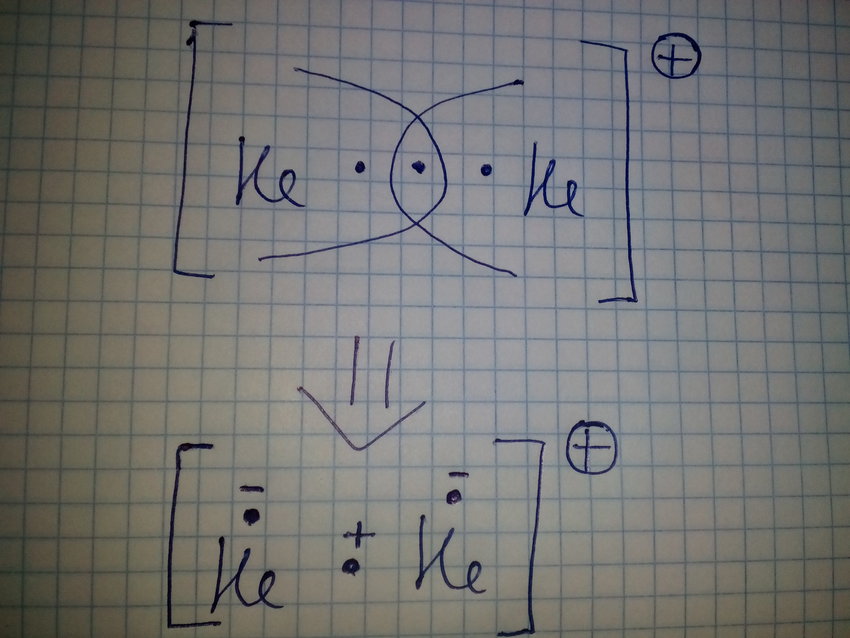
Write The Electronic Configuration Based On Molecular Orbital Theory For The Molecular Orbital Oh Helium He2 Molecule Calculate Its Bond Order And Comment On Its Magnetic Property

Construct The Molecular Orbital Diagram Of He2 Using Appropriate Molecular Orbital Labels And Arrows To Represent Homeworklib

Oneclass Construct The Molecular Orbital Diagram For He2 2 And Sapling Learning Map D Mcanoe Constru
Molecular Orbital Theory Chemistry Encyclopedia Structure Number Molecule Atom Bond Order Multiple Bonds

Use Molecular Orbital Theory To Determine Whether He2 2 Or He2 Is More Stable Draw The Molecular Orbital Diagram For Each And Explain Study Com














0 Response to "45 mo diagram for he2"
Post a Comment