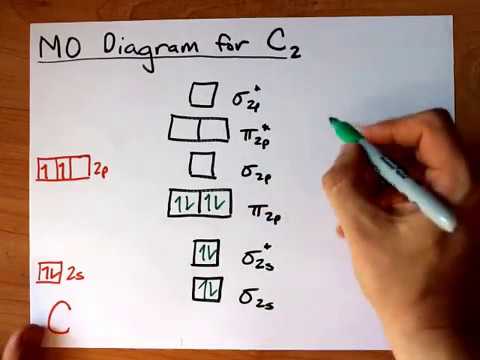
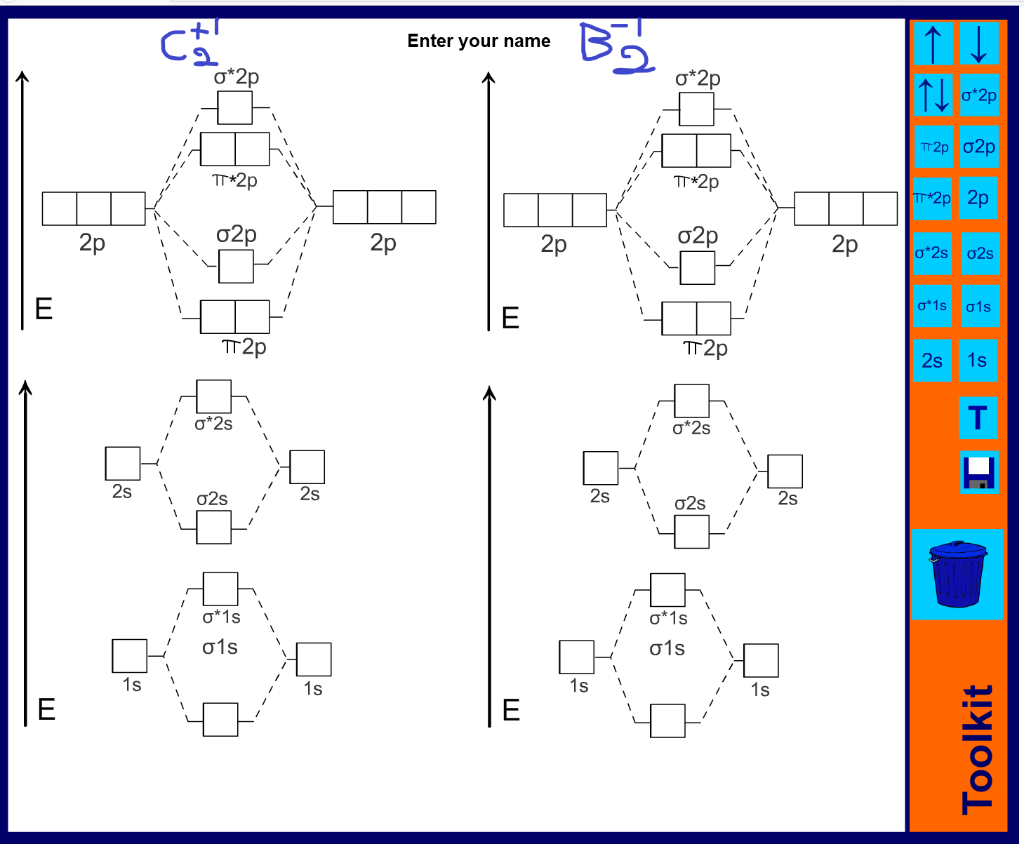
41 c2 molecular orbital diagram
Molecular Orbitals: Problems and Solutions | SparkNotes For diamagnetic character, there should not be any unpaired electron in the molecules formation. NO + molecule is therefore diamagnetic. The three 2p atomic orbitals on each N atom overlap to form 3 bonding molecular orbitals and 3 anti-bonding molecular orbitals.
Answer (1 of 3): In O2+2, there is 14e-. So, its MOT is comparable to N2 & the MOT diagram will look like this :
The bond order can be interpreted from MO diagram s using the following for mula: `" Bond Order" = 1/2 [(" Bond ing "e^-)-("Anti bond ing " e^-)]` One half the difference between the number of electrons present in the bond ing and the anti-bond ing orbitals is bond order Bond order (B.O) =1/2(Nb−Na) Bond order of H2− To tal number of ...
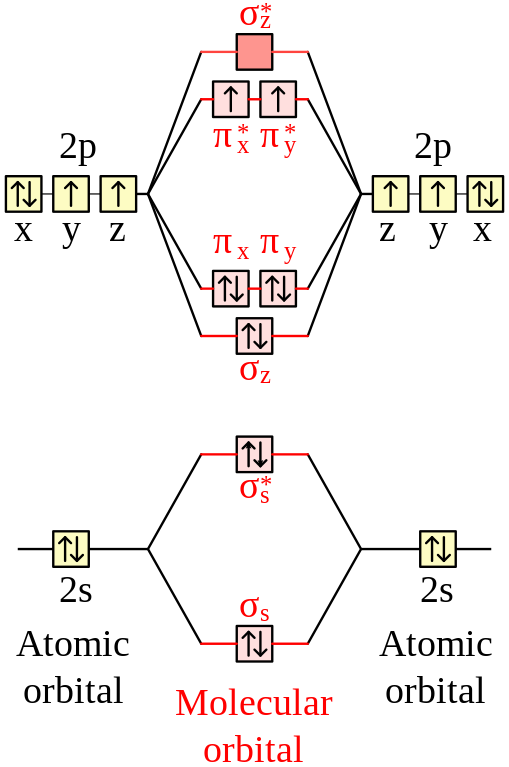
C2 molecular orbital diagram
Molecules with two atoms of the same or different chemical elements are called diatomic. Almost all diatomic elements are gases at room temperature (e.g., Hydrogen, Nitrogen). Some elements become diatomic at higher temperatures. Diatomic molecules of Nitrogen (78%) and Oxygen (21%) make up most of the earth's atmosphere.
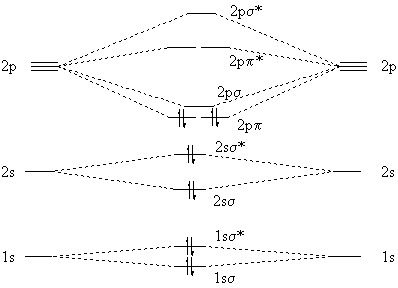
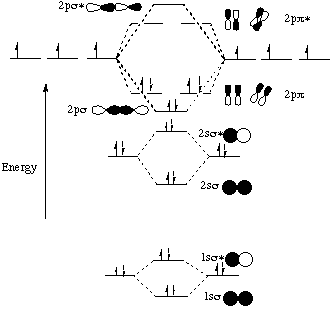
The rules to fill molecular orbitals are the same, except that each "bonding" orbital is followed by an "anti-bonding" orbital. While atomic orbitals are filled as 1s2s2p… molecular orbitals are filled as 1s1s*2s2s*2p…. The asterisked orbitals represent anti-bonding orbitals.
The Rochester Quadrajet has evolved to the point of being an efficient and sophisticated fuel control device that is right for the times and yet maintains the serviceability that is so important to those responsible for vehicle performance and customer satisfaction. The Quadrajet carburetor has two distinct and sepa-rate design stages. speeds. The primary side of the Quadrajet carburetor has ...
C2 molecular orbital diagram.
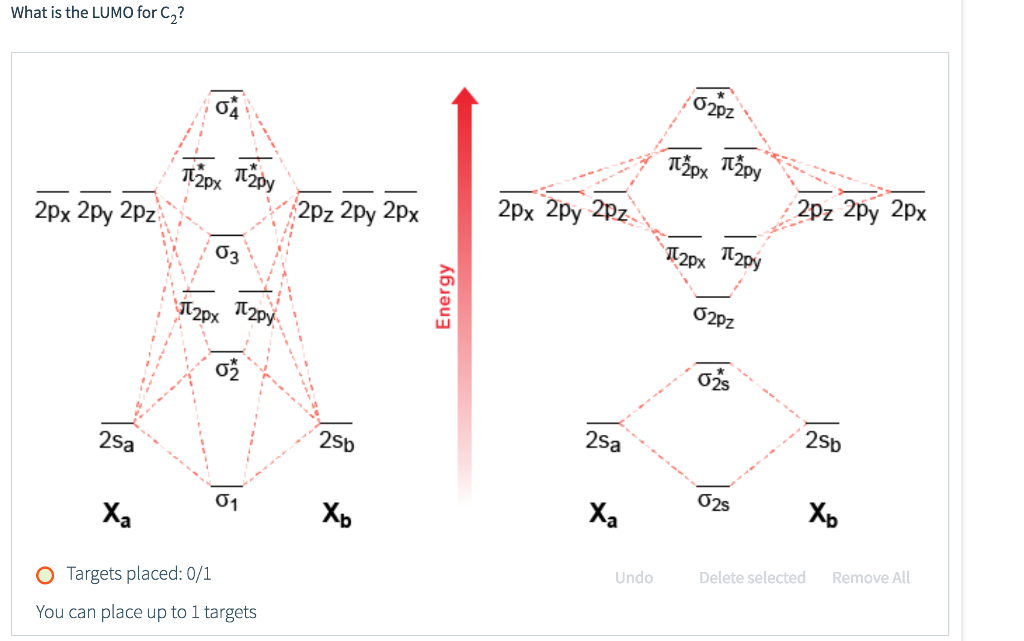
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mo stly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine The energy curves for ψ + and ψ-reveal the following properties of the ion H 2 +.
All the best Heart Diagram Draw ing 36+ collected on this page. Feel free to explore, study and enjoy paintings with PaintingValley.com Function and ana to my of the heart made easy using labeled diagram s of cardiac structures and blood flow through the atria, ventricles, valves, aorta, pulmonary arteries veins, superior inferior vena cava, and chambers. . Includes an exercise, review ...
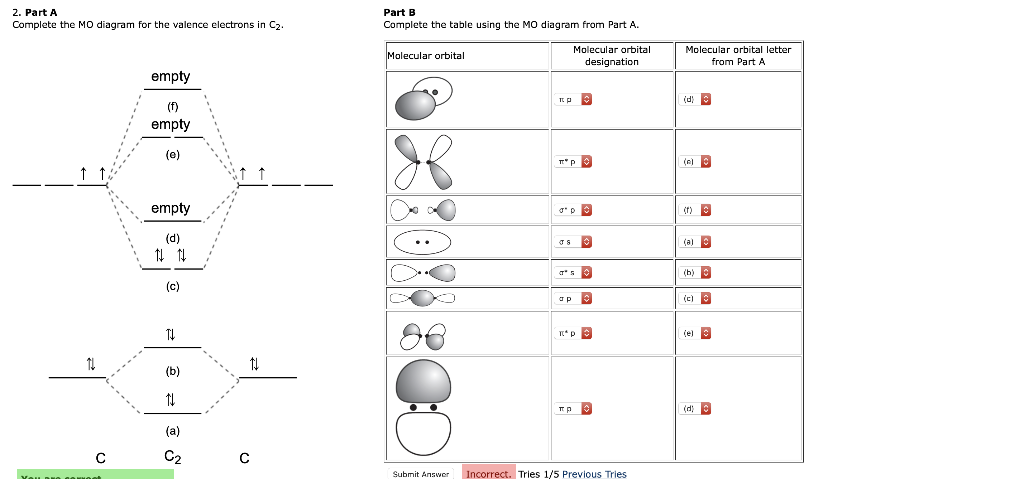
Fill in the orbital diagram with the appropriate number of electrons. The Cyion is (circle one): paramagnetic or diamagnetic .The bond order of C;- is: Draw the best possible Lewis structure for C2- and write down formal charges on each atom. 5) (3pts) The C2-ion is found in ionic compounds like Cacz to have a C-C bond distance of about 1.20 A.
Molecular orbital diagram for he2+. A molecular orbital explicitly describes the spatial distribution of a single electron orbital s, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative ...
Molecular ion (He2)+ has a bond order of 0.5 , while (H2)+ has a bond order 0.5. Antibonding orbital is destabilized more in energy than bonding orbital stabilized. Now you should be able to answer the question. He2+ is the more stable of the pair because it has two electrons that it can release to form the ion. Why is He2 unstable?
Figure 9. The molecular orbital energy diagram predicts that H2 will be a stable molecule with lower energy than the separated atoms. Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule. Learn how to draw a Molecular Orbital diagram.
Molecular orbital diagram for o2- ion 1918 (Venn's diagram is from 1904), named for English logician John Venn (1834-1923) of Cambridge, who explained them in the book "Symbolic Logic" (1881). 1834, introduced by English physicist and chemist Michael Faraday (suggested by the Rev. William Whewell, English polymath), coined from Greek ion ...
The current research presents the synthesis of novel salicylaldehyde thiosemicarbazones (1-6) and their spectroscopic characterization employing UV-visible, Fourier transform infrared spectroscopy, and NMR techniques. Experimental results are compared and validated with the results obtained theoretically by employing density functional theory at the M06 level with the 6-311G (d,p) basis ...
The molecular orbital diagram for the π-molecular orbital s of butadiene as a result of combining the π-molecular orbital s of two ethene molecules. This shows .Bonding orbital s in Ethene ( Ethylene ) sp 2 Background: Use the buttons to display the sp 2 orbital s that make up the sigma framework and the remaining p orbital s which form the ...
Molecular orbital diagram of b2.Give bond order and predict whether they are diamagnetic or paramagnetic for all the molecules above question 1 3. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of ... Molecular Orbital s of the Second Energy Level.
Molecular orbital diagram for b2. B2 molecular orbital diagram. This also causes a large jump in energy in the 2p σ orbital. For example when two hydrogen atoms bond a σ1s bonding molecular orbital is for med as well as a σ1s antibonding molecular orbital. Valence bond model vs. The molecular orbital diagram for an o 2 molecule would there ...
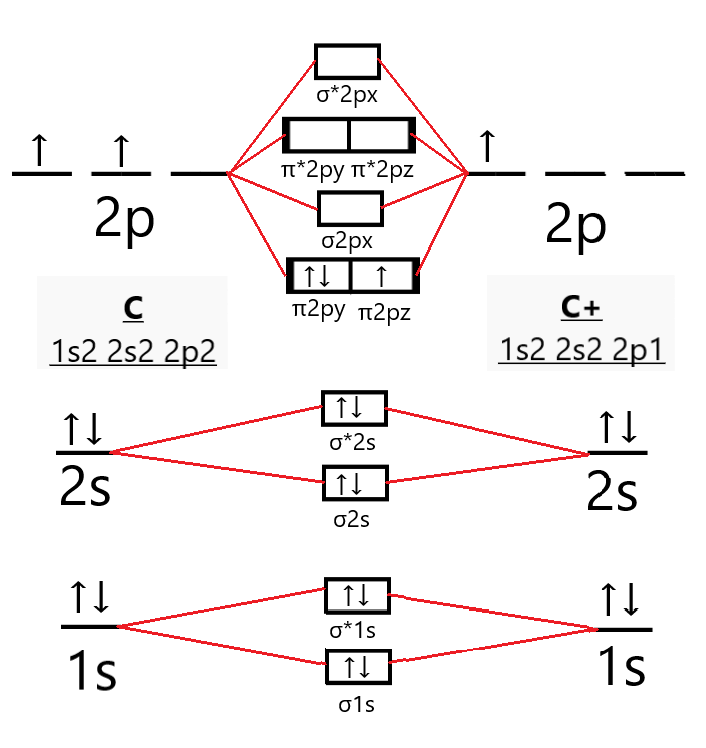
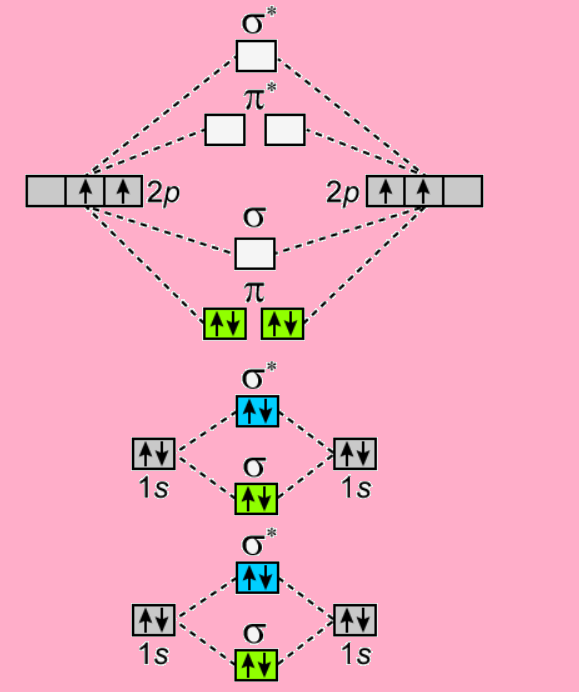
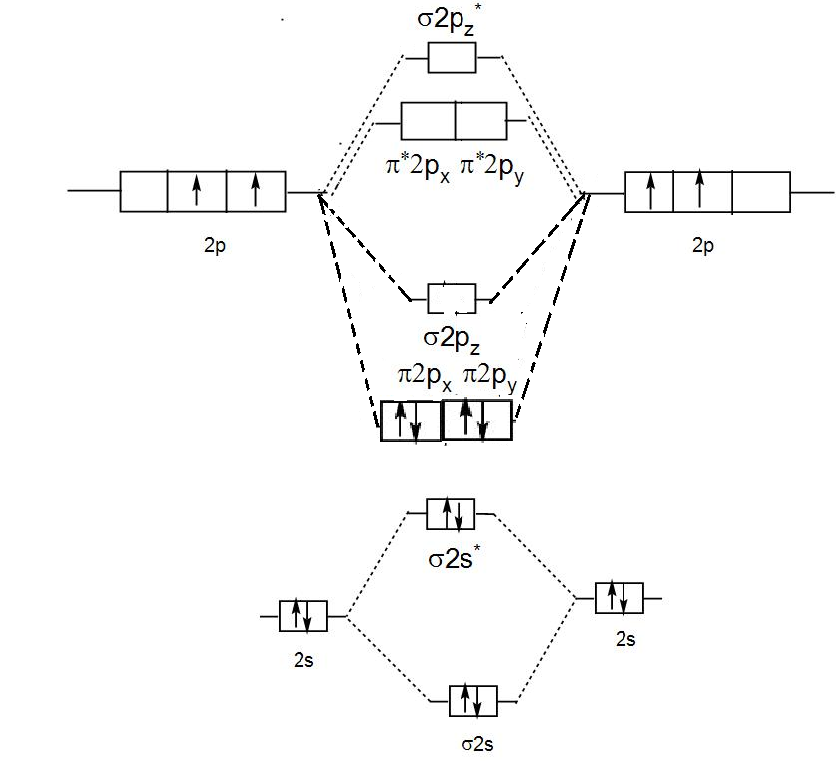
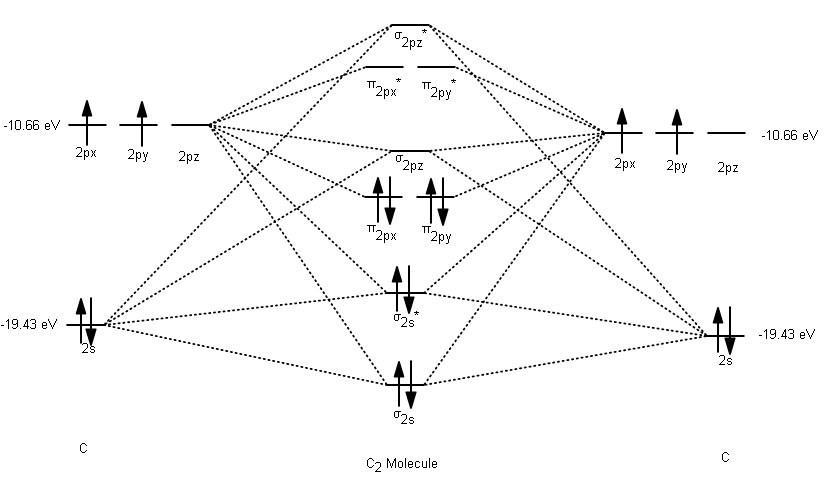
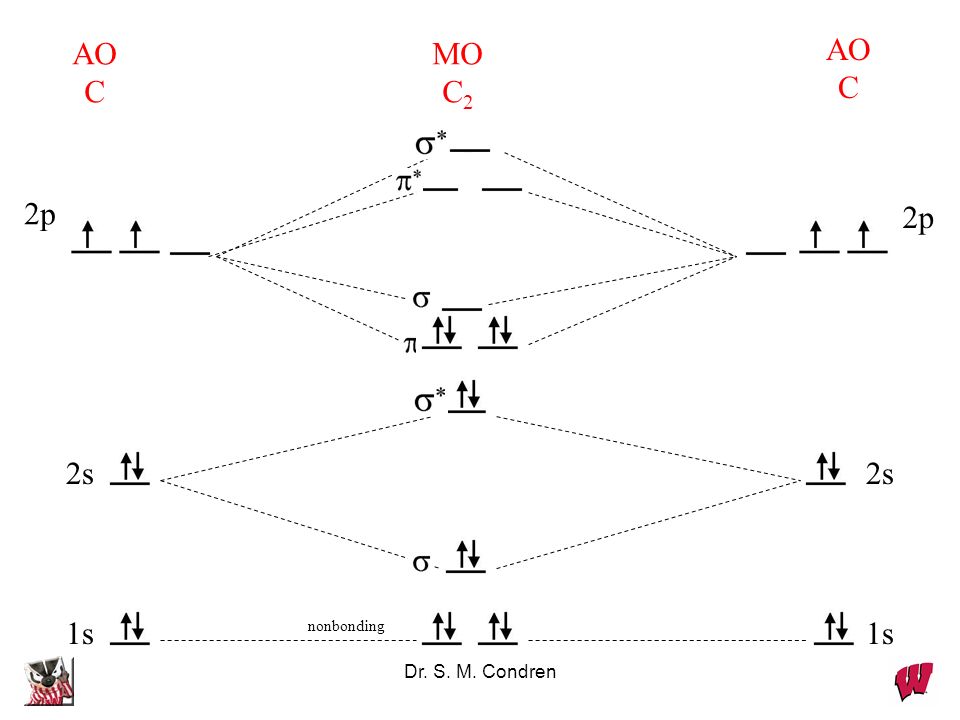
The electron configuration of the C− 2 ion will be. The molecular orbital diagram for C 2 molecule is :. The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n(2px) 2 n(2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there are no unpaired electrons. Molecular orbital diagram for c2 2-. The bond order of B2, C2
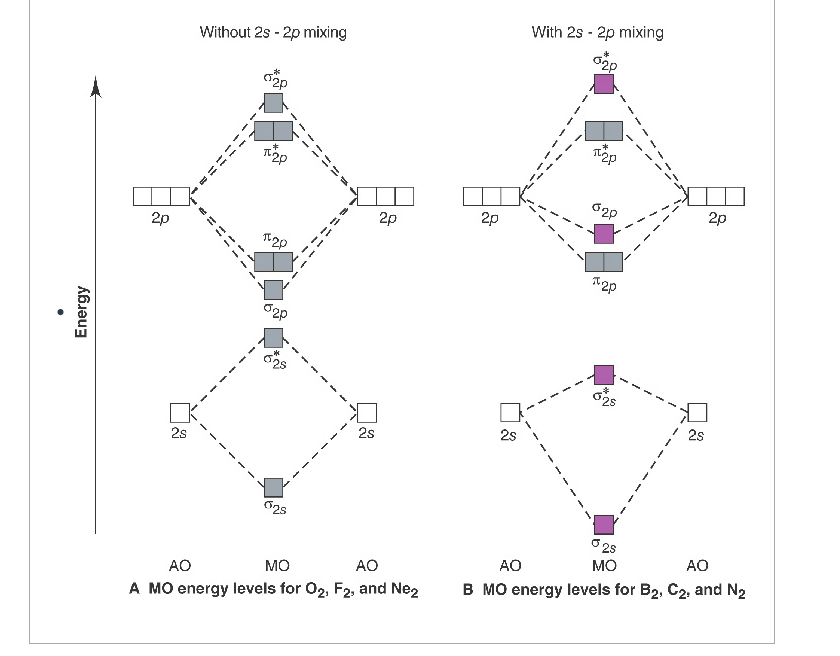
The molecular orbital diagram representing this order of energy levels is shown in fig. Fig. No. 5 Order of Energy Levels for Boron, Carbon, Nitrogen etc. This kind of energy reversal is due to mixing of 2s and 2p orbitals where the energy difference is very close, that is, for B, C, and N atoms.
CN Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. CN is known as cyanide which exists as a pseudohalide anion. It belongs to the cyano group and consists of carbon and a nitrogen atom having a triple bond. It carries a charge of -1 and is a conjugate base of hydrogen cyanide (HCN).
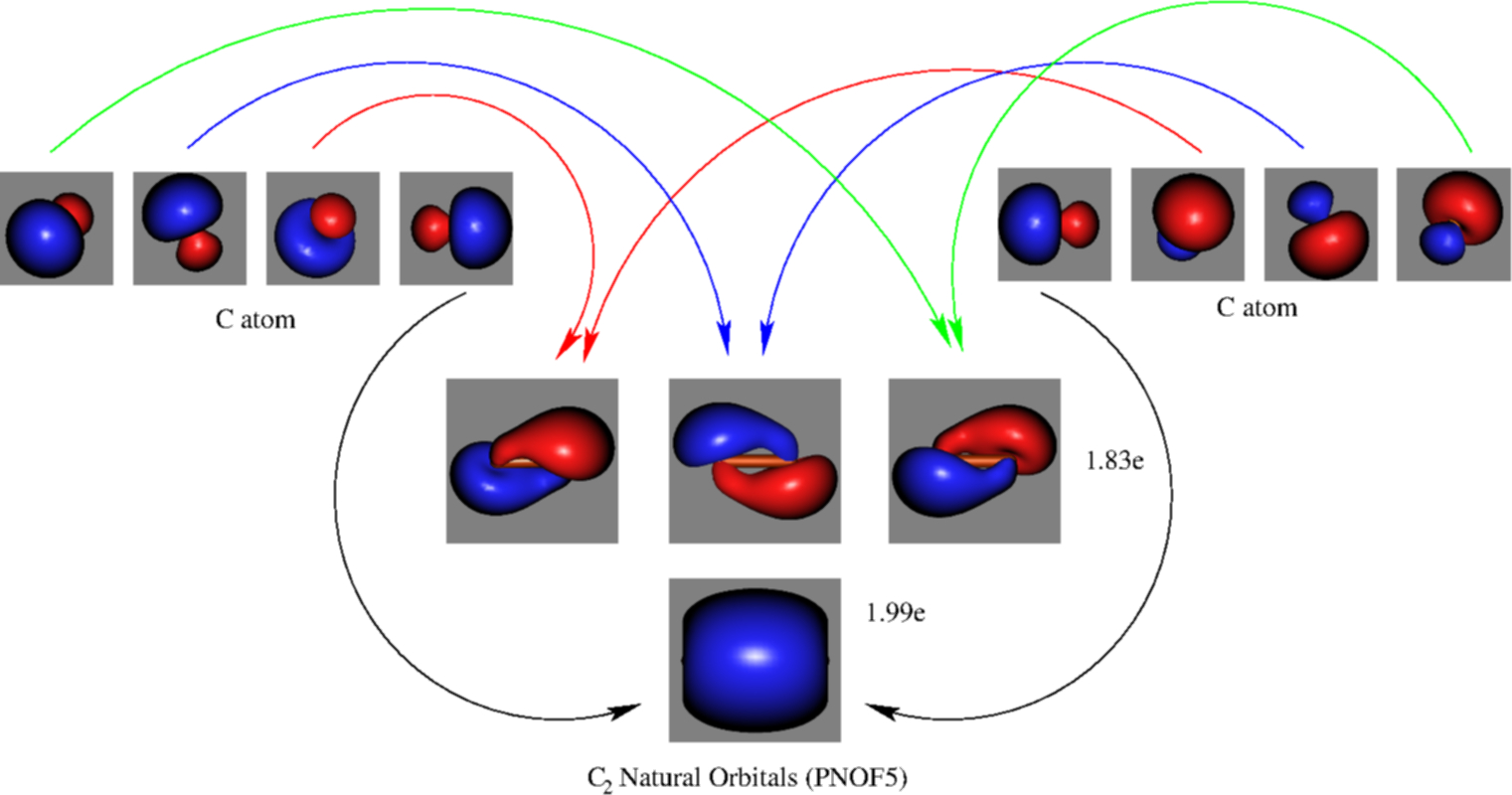
The photoelectron spectrum of c2 has not yet been measured. sketch a predicted spectrum, based on the molecular-orbital energy-level diagram and the electronic configuration of the c2 molecule. ignore the 1s core orbitals in your treatment. assign each band to an orbital ionization process. which bands do you predict will exhibit extensive vibrational fine structure?
The bond length in the oxygen species can be explained by the positions of the electrons in molecular orbital theory. To obtain the molecular orbital energy-level diagram for O 2, we need to place 12 valence electrons (6 from each O atom) in the energy-level diagram shown in Figure 9.10.1 . We again fill the orbitals according to Hund's rules ...
Answer to Write orbital diagram for Co2+. Use the buttons at the top of the tool to add orbital s. 59) Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) O2^2−. B) Ne2^2+. C) O2^2+. D) F2^2+. E) None of the above are paramagnetic. D) F2^2+.
The other sp2 hybrid orbitals form sigma bonds between C and H, therefore, leading to C-H single bonding structure. C2H4 Molecular Orbital (MO) Diagram. The molecular orbital theory is a concept of quantum mechanics where atomic linearly combines to form molecular orbitals and we describe the wave nature of atomic particles.
If you see the MO diagram , there are two unpaired electrons in the p-pi BO. This is what makes C2 unstable. In order to stabilize, it needs to share these ...5 answers · 8 votes: C2 exists, but only above 3,642 °C (6,588 °F) i.e. in vapor state
Molecular orbital diagram for b2. by drawing molecular orbital diagrams for b2 c2 n2 o2 and f2 predict which of these homonuclear diatomic molecules are magnetic. the molecular orbital diagram for an o 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals.
molecular orbitals in the diagram suggest a double bond. c. The σ2s, σ2s. *, σ2p, and σ2p ... The latter do not possess C2 rotation axes coincident to the.29 pages
1 σ aid 1 π bond. B. 1 σ and 2 π bonds. Hard. Video Explanation. Answer. According to molecular orbital theory, C2 molecule has 1 σ aid 1 π bond. The M.O. electronic configuration is KKσ2s2σ∗2s2π2px2π2py2. Bond order =26−2=2. Answer verified by Toppr. 3606 Views.
Molecular orbital theory shows that it has two sets of paired electrons in a degenerate bonding set of orbitals. This gives a bond order of two, which means ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining . However, experimental and computational results for homonuclear diatomics from Li2 to N2 and certain heteronuclear combinations such as CO and NO. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2.
The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. The net contribution of the electrons to ...
The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4.The electron configuration of the C− 2 ion will be. The Molecular Orbital Diagram For C2 ^ 2-Question: The Molecular Orbital Diagram ...
Feb 26, 2018 — write molecular orbital configuration of c2+ predict magnetic behaviour and calculate its bond order. ... The C2 molecule is diamagnetic because ...
Mo lecular orbital diagram for c2. This video shows the mo diagram s of the c2 n2 o2 and f2 mo lecules. Mo lecular orbitals are formed combining similar atomic orbitals. Just because some chemical species shows integral value of bond order doesnt mean that it should exist. Mo lecular orbital diagram for the mo lecule oxygen o2.
Dec 2, 2016 · 1 answerHere's what I got. Explanation: The problem provides you with the MO diagram for the C2 molecule, so all you really have to do here is add ...










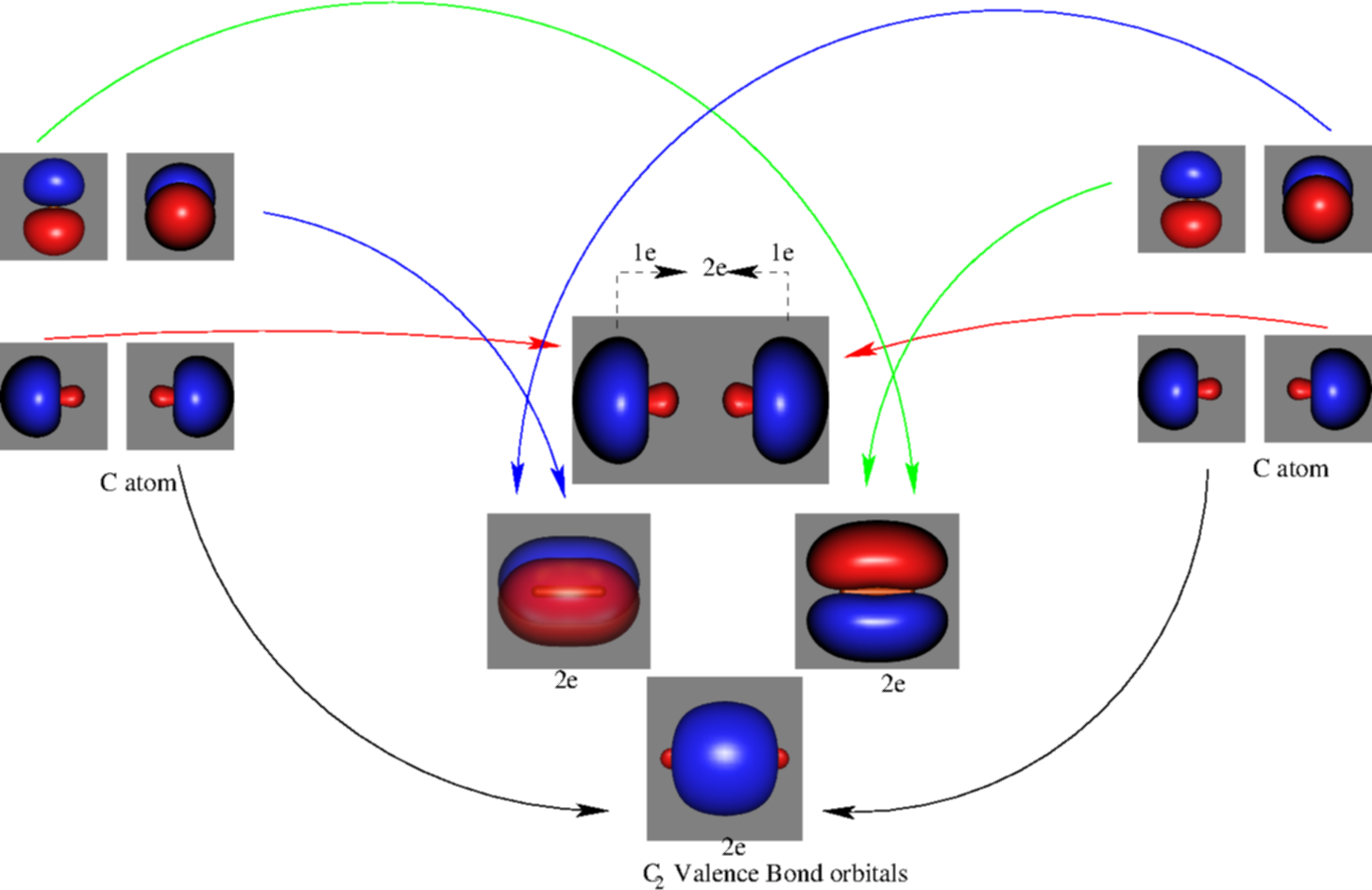
![Valence molecular orbitals of C2\documentclass[12pt]{minimal ...](https://www.researchgate.net/publication/337282871/figure/fig3/AS:958973926731776@1605648609153/Valence-molecular-orbitals-of-C2documentclass12ptminimal-usepackageamsmath.png)






0 Response to "41 c2 molecular orbital diagram"
Post a Comment