41 lewis dot diagram for n2
NO2 (Nitrogen Dioxide) Lewis Dot Structure. Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3.
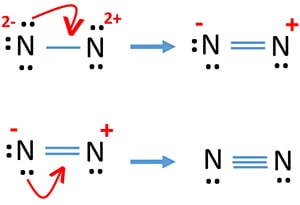
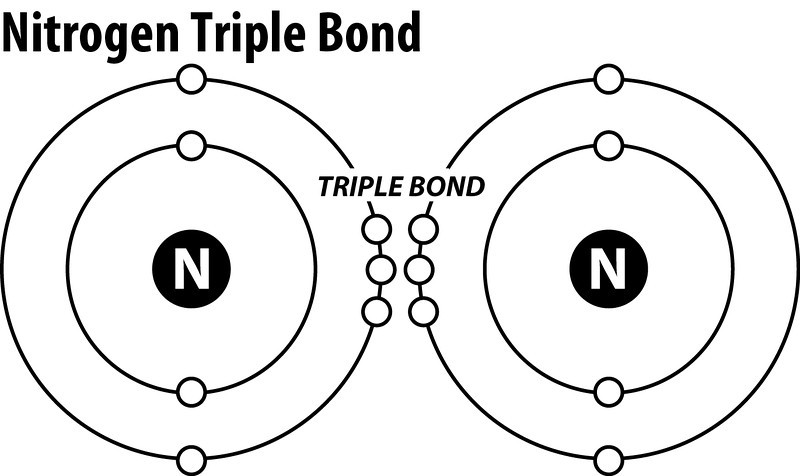
Answer (1 of 3): Plucked this image from google. There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple covalent bonds. The remaining lone pairs are repulsed by this dense electronegativity and so are drawn to appear as far away as possible.
Lewis Dot Diagram for Nitrogen. what is lewis dot diagram of nitrogen gas answers the lewis dot structure of a nitrogen atom would be the capitol letter n with the five valence electrons represented by two dots above it one to the lewis dot structure for nitrogen atom n a step by step explanation of how to draw the lewis dot structure for n nitrogen i show you where nitrogen is on the periodic ...
Lewis dot diagram for n2
Answered: Draw the Lewis Dot Structure for N2.… | bartleby. Draw the Lewis Dot Structure for N2. What type of bond keeps this molecule together (single, double, or triple)? Is the bond polar or nonpolar and why?
Lewis dot diagram for n2. Each nitrogen atom also has a lone pair of electrons. The remaining lone pairs are repulsed by this dense electronegativity and so are drawn to appear as far away as possible. The lewis dot structure for any molecule can be found by following a general set of rules consisting of 5 or sometimes 6 steps.
The Lewis dot structure for Nitrogen will be this: However, when two atoms of Nitrogen bind together, it has the following structure: Here both these atoms share six valence electrons to form a triple bond. These electrons are shared equally, and both Nitrogen atoms have one lone pair of electrons. N2 Polarity
Lewis dot diagram for n2.
1. Draw lewis dot structures for O2 and N2 se the density of dry air to calculate the mass, in grams, of a 240 mL sample of air at a pressure of 750 mmHg and a temperature of 19°C Refer to the chart in the lab techniques section for dry air density in units of E/L An unknown gas sample is collected in a 255 mL container at 25'C and 755 mmHg.
The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it, one to the left right and bottom of it. .. . N . .
Aug 22, 2021 · The N2 Lewis structure has a triple bond between two nitrogen atoms. It's perfectly symmetric. According to the octet rule, nitrogen atoms need to bond three times. In a Lewis structure, the nucleus of the element is represented by its symbol. is 3. Popular Questions of Class 11 Chemistry. /hybrids.
N2 Lewis Structure The N2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair. N2 Lewis Structure Setup It's easiest to think in terms of […]
The following is the Lewis Dot Structure for N2, 2N and N2, are basically two forms of the same element. There is a little difference between the two. 2N refers to two molecules of Nitrogen atom, and N2 states that two atoms of Nitrogen are present in a single molecule. The number written at the start, refers to the number of molecules and the ...
Answer (1 of 2): In exactly the same way as you'd do it for any simple, covalently bonded compound. First you need to know the valency of each element. Then you need to draw a stick model with bonds between the atoms, so that each atom's valency is satisfied (so N has three bonds coming out of i...
A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t...
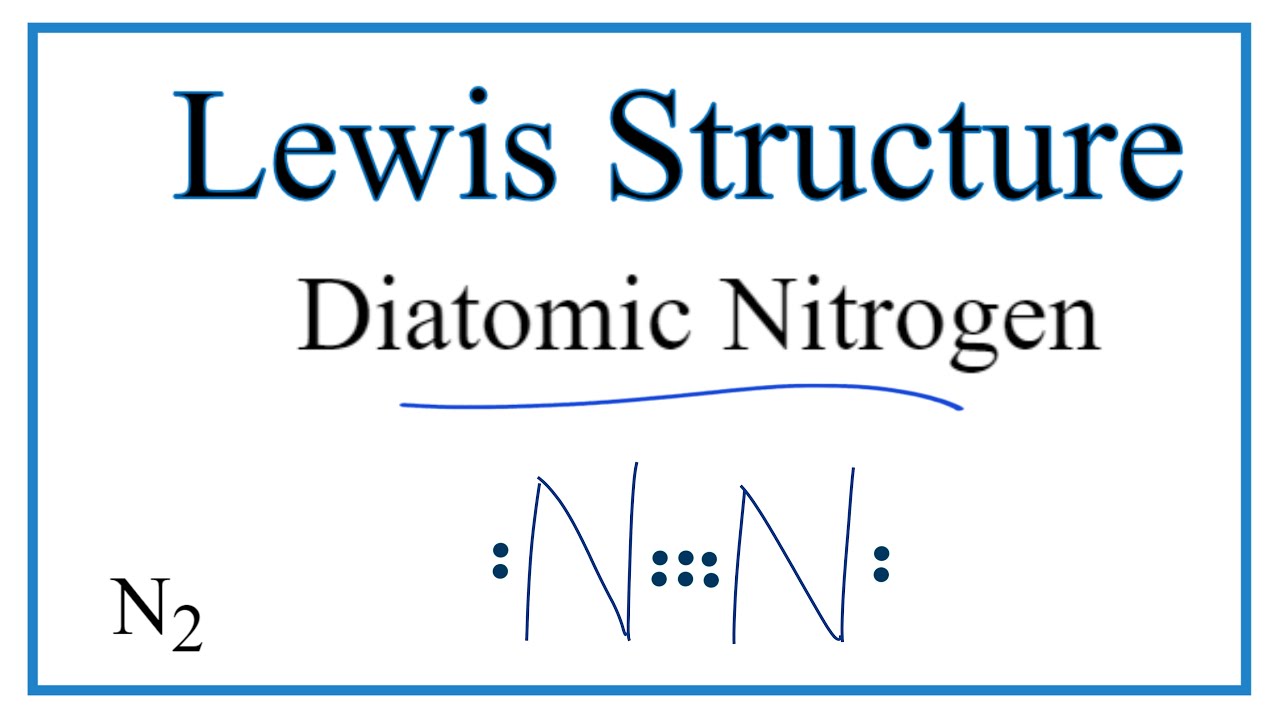
Transcript: For the N2 Lewis structure, we have five valence electrons for Nitrogen--it's in group 5 or 15 on the periodic table. We have two Nitrogens. Multiply those together, we have a total of 10 valence electrons for the N2 Lewis structure. We'll put the two Nitrogens next to each other, and then we'll put two valence electrons between them to form a chemical bond.
In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. Here is the electron dot structure for a single N atom: The total number of valence electrons between the two N atoms is 10 e^-.
on Lewis Dot Diagram For N2. Plucked this image from google. There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple. The Lewis Structure for N2 looks easy at first. The problem is that there aren't enough valence electons to give both Nitrogen atoms an octet. You'll need to use .
In the Lewis structure of the N2 molecule, over there is a formation of a triple covalent bond stood for by three lines between two atom of Nitrogen. The leftover 2 2p orbitals become two π bonds and electrons making a pair in between the nitrogen atoms will make a sigma bond.
Atomic nitrogen has 5 valence electrons and 4 valence orbitals (2s, 2px, 2py, and 2pz). In the Lewis structure there is a triple bond between the nitrogen atoms and non-bonding pair of electrons ...
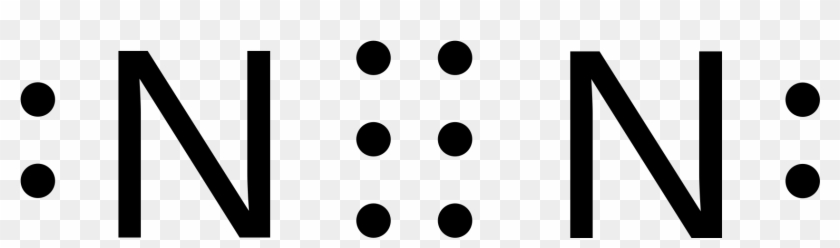
What is the Lewis dot diagram for nitrogen? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable. The two letter N's in the N2 Lewis structure represent the nuclei (centers) of the ...
Lewis dot structure for n2. If you are talking about the Lewis Dot Diagram then N 2 would have 5 dots around each of the letter Ns so that there would be 6 dots total What is the Lewis dot structure for N2 look like. The shape of a molecule. How the molecule might react with other molecules.
Lewis dot structure of n2 molecule Let's take a look at the Lewis of and N2 structure. Atomic nitrogen has 5 valence electrons and 4 orbital valence (2s, 2 px, 2py and 2pcs). In the Lewis structure there is a triple link between nitrogen atoms and a pair of non-binding electrons on each. This is consistent with the physical properties of N2.
The N2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair.
Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell. As per the molecule N2, it has two atoms of Nitrogen.
How to Draw the Lewis Structure of N2 - with explanation!Check me out: http://www.chemistnate.com
Draw the Lewis dot structure for N{eq}_2 {/eq} and a second structure showing the bonds and the VSEPR shape. What is the name of the shape? How many lone pairs of electrons are present?
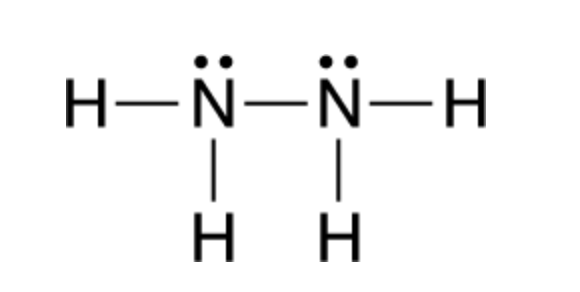
What is the Lewis dot structure for N2H2? In the N2H2 Lewis structure the two Nitrogen (N) atoms go in the center (Hydrogen always goes on the outside). Hydrogen (H) only needs two valence electrons to have a full outer shell. In the Lewis structure for N2H2 there are a total of 12 valence electrons.
Answer: N2 Lewis structure (nitrogen electron dot structure) is that type of structure where we show the total ten valence electrons of N2 as dots , or dots and dashes (-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash (-) , or dots ( ) but a lone pair of two electrons is shown by dots [ ]. In N2 Lewis ...
Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell.As per the molecule N2, it has two atoms of Nitrogen.






















![Draw the electron dot structure of Nitrogen molecule [N = 7]](https://d1hj4to4g9ba46.cloudfront.net/questions/1890007_1909574_ans_16e2a124f2974b5694de1a9f3c97eebd.png)





0 Response to "41 lewis dot diagram for n2"
Post a Comment