41 orbital diagram of argon
Argon provides an inert atmosphere in which welded metals will not oxidise. Appearance. Argon is a colourless, odourless gas that is totally inert to other substances. Uses. Argon is often used when an inert atmosphere is needed. It is used in this way for the production of titanium and other reactive elements. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represent the electrons in ...
The orbital diagrams below are of argon, nitrogen, and electron. image via galleryhip.com. image via chemistryland.com. image via chemwiki.ucdavis.edu. Unlike an s orbital, a p orbital points in a particular direction - the one drawn points up and down the page. At any one energy level it is possible to have three absolutely equivalent p ...

Orbital diagram of argon
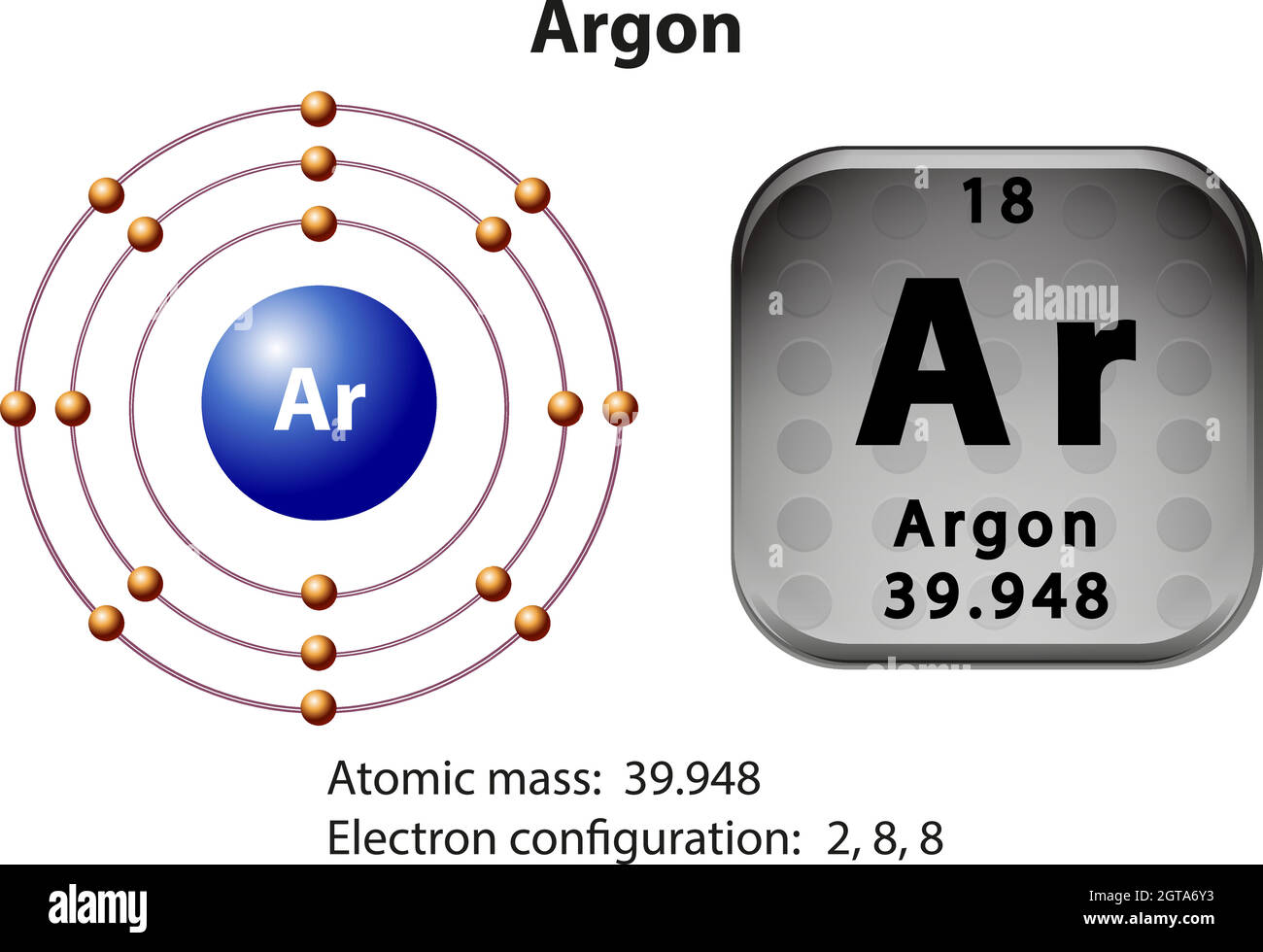
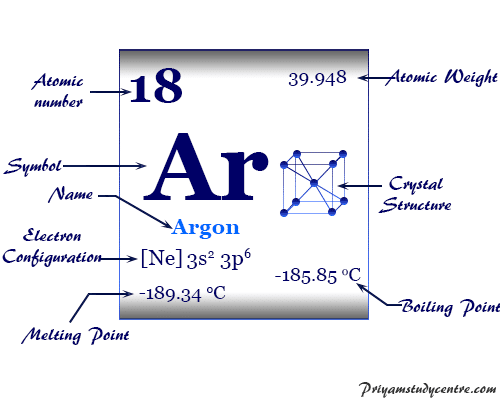
Density: 0.00166 g/cm 3 . Electronic configuration of the Argon atom: 1s 2 2s 2 2p 6 3s 2 3p 6. Reduced electronic configuration Ar: [Ne] 3s 2 3p 6. Below is the electronic diagram of the Argon atom Distribution of electrons over energy levels in the Ar atom. 1-st level (K): 2. 2-st level (L): 8. 3-st level (M): 8. On a mission to a newly discovered planet, an astronaut finds chlorine abundances of 13.85% for 35Cl and 86.15% for 37Cl. The following is an orbital diagram for a nitrogen atom. Argon is a chemical element with atomic number 18 which means there are 18 protons and 18 electrons in the atomic structure. Give the orbital diagram for the element with the electron configuration {eq}1s^22s^22p^63s^23p^6 {/eq}. Argon: Argon is a noble gas and therefore not reactive like other elements due to its full ...
Orbital diagram of argon. What is Orbital Welding.. When high quality results are required, orbital welding is the first choice for connecting pipes. The welding torch - in most cases, TIG welding (Tungsten Inert Gas) is used - travels around the pipes to be connected, guided by a mechanical system. The name orbital welding comes of the circular motion of welding tool ... Orbital diagram of Argon (Ar) 19: Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of ... The electronic configuration of Argon (atomic number is 18) is- 1s^2 2s^2 2p^6 3s^2 3p^6 Note:- For writing the electronic configuration of elements, the Aufbau Principle is used. In Aufbau Principle, the electrons are filled according to the increasing energy level of orbitals. According to the Aufbau Principle, first the atomic number of element is determined (like here oxygen has atomic ... Earlier I was listening to [a podcast about neutrinos](http://www.bbc.co.uk/programmes/b0106tjc), and at one point someone mentions looking for argon atoms amongst [...a different kind, let's say helium for simplicity's sake] atoms as proof of something. I remember those diagrams in my text books, with the little nucleus and the orbiting electrons, but obviously that's not what you see under a scanning idontknowtheterm microscope or whatever the technology is. What do you see when you look at an...
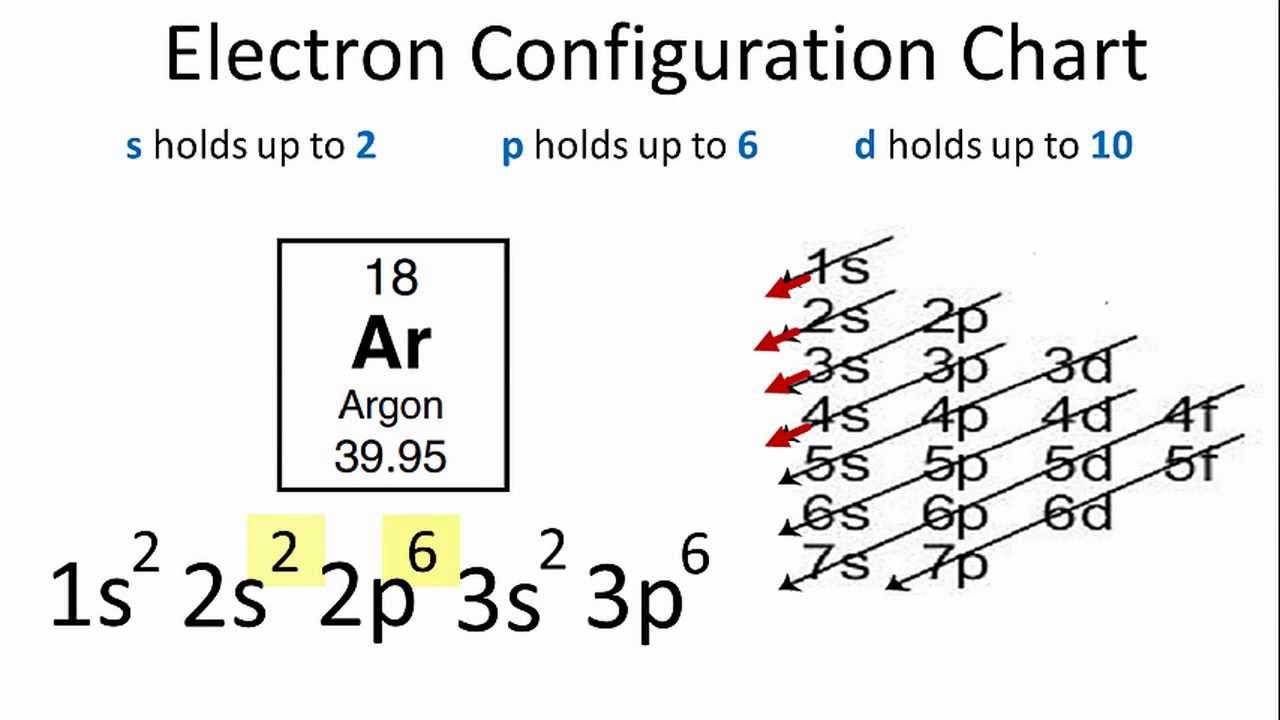
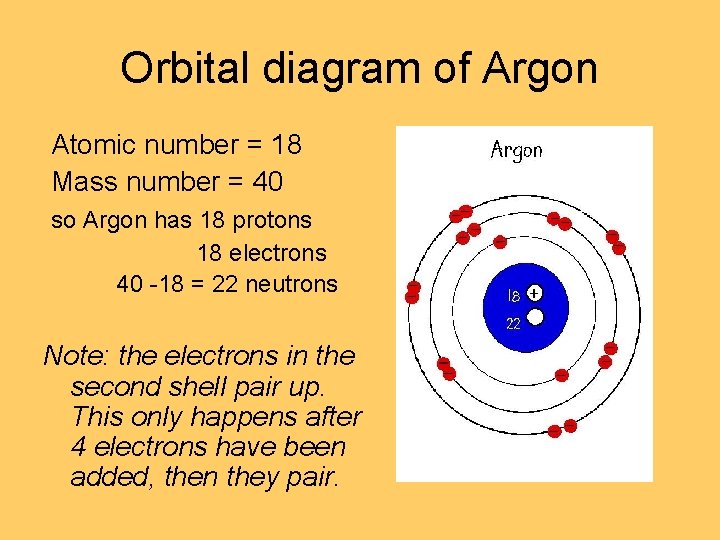
In order to write the Argon electron configuration we first need to know the number of electrons for the Ar atom (there are 18 electrons). When we write the configuration we'll put all 18 electrons in orbitals around the nucleus of the Argon atom. In writing the electron configuration for Argon the first two electrons will go in the 1s orbital. 24 Jan 2021 — Argon's electron configuration for the first two electrons goes in the 1s orbital. 1s only hold two electrons and the next 2 electrons for Argon ... In an orbital filling diagram, the individual orbitals are shown as circles (or squares) ... Since 1s can only hold two electrons the next 2 electrons for Argon go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. The sequence of orbitals for electron configuration can be seen from this diagram. The maximum number of electrons that can be filled in each orbital are: s = 2.1 answer · Top answer: [readmore]Hey there! We have to draw the electron configuration for a neutron atom of argon.The electron configuration of an atom or molecule ...
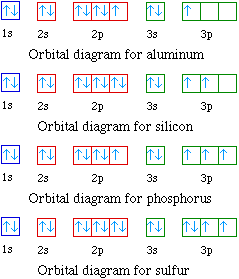
Original prompt: [[WP] Bryan Wetherspoon, 26, an interstellar pilot, has just been made redundant owing to increased automation. Vengeful, he is now enroute to Persephone in 40 Eridani B to blow up Jupiter Command, the central Hive Mind of all AI systems across the human interstellar community.](https://www.reddit.com/r/WritingPrompts/comments/oxo78o/wp_bryan_wetherspoon_26_an_interstellar_pilot_has/) The talking head continued screaming, broadcasting his fury across *NeuroNet*. "Even...eve... When we write the configuration we'll put all 18 electrons in orbitals around the nucleus of the Argon atom. In writing the electron configuration for Argon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Argon go in the 2s orbital. Orbital Diagram For Aluminum.They consist of the symbol for the element in the. It explains how to write the orbital diagram. Electron Dot Diagram For Aluminum — UNTPIKAPPS (Millie Bailey) Here are some orbital diagrams of elements with more electrons to help you understand the rules, electron configuration, orbital diagrams, and quantum numbers. . They consist of the symbol for the element in To write the orbital diagram for the Argon (Ar) first we need to write the electron configuration for just Ar. To do that we need to find the number of elec...
Chem4Kids.com: Argon: Orbital and Bonding Info. Check out the blackboard. That box on the left has all of the information you need to know about one element. It tells you the mass of one atom, how many pieces are inside, and where it should be placed on the periodic table . In the next section we're going to cover electron orbitals or electron ...
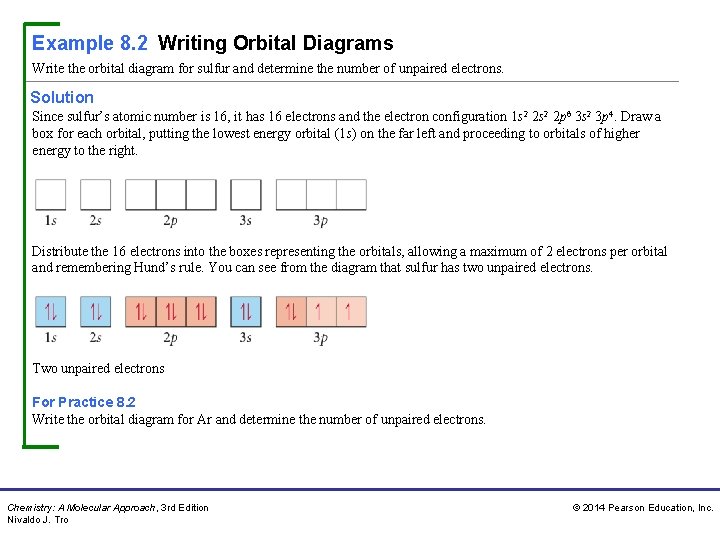
Write the complete orbital configuration for argon. Electronic Configuration: The electronic configuration of an atom shows the packing of electrons in the electron orbitals.
Each box represents an orbital. Each orbital can hold up to two electrons. Ar. Question: Part A - Write the electron configuration for the Argon atom. Ch6: Photoelectron Spectroscopy. Seg1 Orbitals and Multi-Electron Atoms 114-OL- Course The electron energy-level diagram below shows the relative energies of orbitals for any multi- electron atom.
Argon (Ar) has an atomic mass of 18. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
Start studying Unit 3: Orbital Diagrams. Electron configuration for Kr (Krypton). 1s2 2s2 2p6 3s2 What element is represented by this orbital diagram?.In writing the electron configuration for Argon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Argon go in the 2s orbital.
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
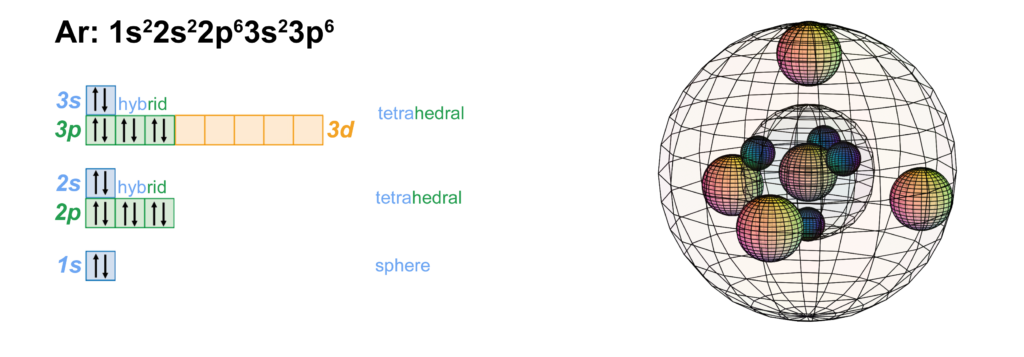
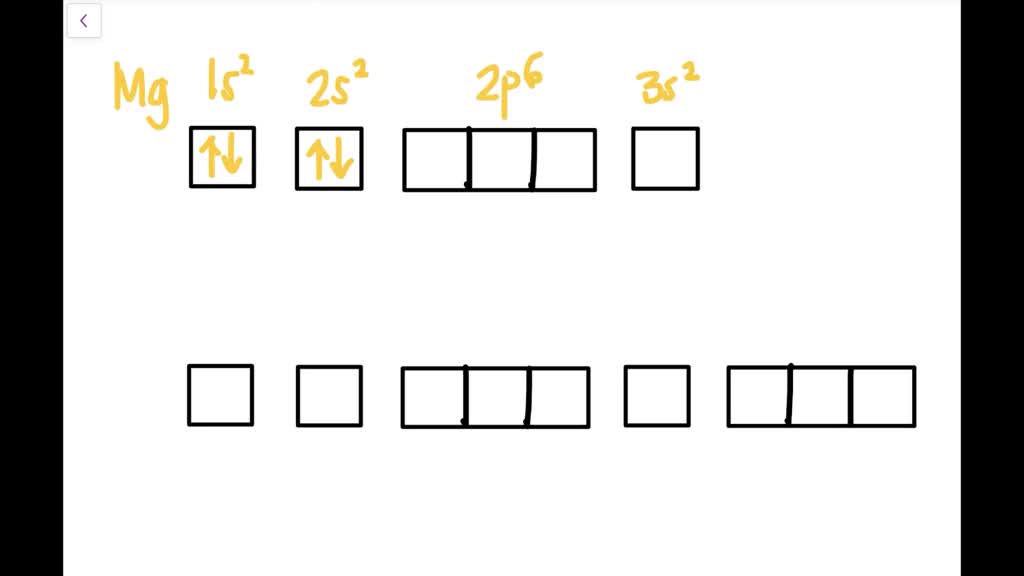
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
2.The orbital notation of an atom in the ground state is A)C B)N C)B D)Be ... 13.The diagram below represents the orbital notation of an ... 14.Which is the orbital notation for the electrons in the third principal energy level of an argon atom in the ground state? Answer Key Electon Configurations - Honors Page 3 1. A 2. D 3. A 4. C 5. B 6. D ...
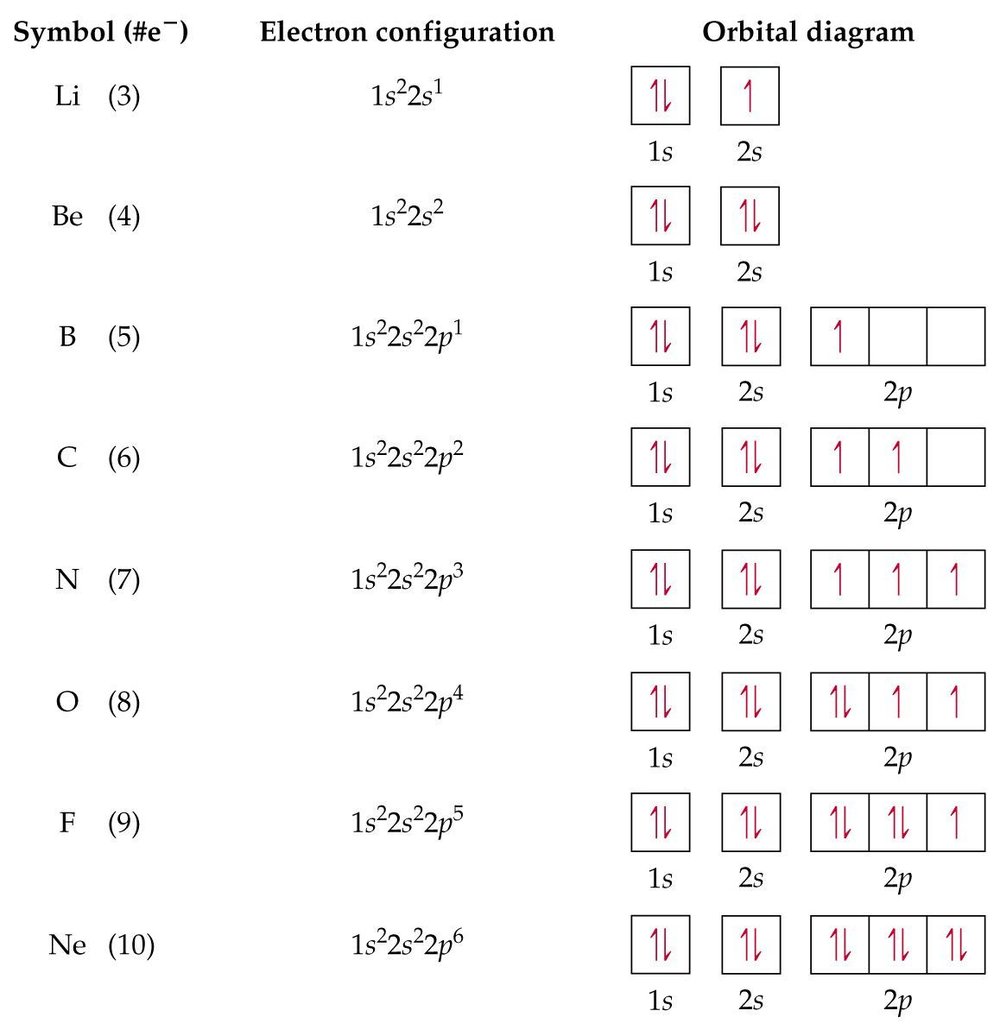
The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 8.3. 3 or 8.3. 4 ). Thus, the electron configuration and orbital diagram of lithium are:
Answer (1 of 5): It is a partial question and the the two answers already given are partial answers. Argon's atomic number is 18 and there are 18 electrons in each atom. Under appropriate physical conditions one can make all the 18 electrons unpaired or 16, 14, 12, 10, 8, 6, 4, 2 electrons unpair...
This video shows how to create an orbital diagram of an atom from its electronic configuration
The electron configuration for argon is : Ar 1s 2 2s 2 2p 6 3s 2 3p 6. The electron configuration for potassium is: ... Orbital Diagrams An orbital diagram is a sketch which shows electron population in atomic orbitals with the electron's spin indicated by up and down arrows.
Orbital Diagram For Arsenic. Because the 4p section has 3 orbitals, but Arsenic ends with 4p3. It'll want to leave as few orbitals empty, so you have three arrows pointing up. The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org!
Orbital Diagram . Orbital Diagrams give a more complete indication of the electron quantum numbers. Each orbital represented by a box and each electron by a half-arrow. ... Let's look at Argon, which has 18 electrons. It has the configuration. 1s 2 2s 2 2p 6 3s 2 3p 6. Now you might be tempted for Potassium (the next element) to put the extra ...
Orbital diagram. Tags: Question 12. SURVEY. 300 seconds. Q. The electron configuration of an atom is 1s 2 2s 2 2p 6. The number of electrons in the atom is. answer choices.
Krypton Orbital Diagram. Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of krypton (atomic number: 36), the most common . Box spin diagram of outer electron orbitals for the electron configuration of the atom . 36 Krypton, Kr, [Ar]3ds24p6 = [Kr] (), [Ar]3d 4s 4p v. stable, Kr .
Give the orbital diagram for the element with the electron configuration {eq}1s^22s^22p^63s^23p^6 {/eq}. Argon: Argon is a noble gas and therefore not reactive like other elements due to its full ...
On a mission to a newly discovered planet, an astronaut finds chlorine abundances of 13.85% for 35Cl and 86.15% for 37Cl. The following is an orbital diagram for a nitrogen atom. Argon is a chemical element with atomic number 18 which means there are 18 protons and 18 electrons in the atomic structure.
Density: 0.00166 g/cm 3 . Electronic configuration of the Argon atom: 1s 2 2s 2 2p 6 3s 2 3p 6. Reduced electronic configuration Ar: [Ne] 3s 2 3p 6. Below is the electronic diagram of the Argon atom Distribution of electrons over energy levels in the Ar atom. 1-st level (K): 2. 2-st level (L): 8. 3-st level (M): 8.
























0 Response to "41 orbital diagram of argon"
Post a Comment