41 square planar orbital diagram
Predict the number of unpaired electrons for each of the following: a. a tetrahedral d6 ion b. \[Co(H2O)6\] 2+ c. \[Cr(H2O)6\] 3+ d. a square-planar d7 ion I'm having trouble solving this type of problem. I have the following questions regarding the answers: 1) How did they determine what was low spin and high spin? For B, I'm puzzled as H2O falls in the grey zone of the spectrochemical series (according to my professors notes, but I have seen sources that don't agree), so it could ... Splitting of the energy of the d-orbitals in square planar transition metal complexes — A general d-orbital splitting diagram for square planar ...
This was the last question on an exam I just took. Skimmed the question in a hurry, just wrote down some bullshit about how Zn^2+ is d8 therefore square planar is most likely, then I regurgitated some tangentially related gibberish about coordination numbers and came out with a worthless answer with no time left on the clock. Now that I'm not rushing it, I'm thinking that I should have calculated the changes in CFSE (or shit, LFSE maybe? Is that possible? god damn it) for the different geometri...
Square planar orbital diagram
form tetrahedral, square planar or pyramidal geometries. MOT for the tetra coordinated complexes can be utilized to construct the molecular orbital diagrams ...12 pages We find that the square planar complexes have the greatest crystal field splitting ligand field (left diagram) and the tetrahedral field (right diagram).D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns: 1. Octahedral 2. Tetrahedral 3. If I wore a stretchy wrist band with magnets on the inside, pressed up against my skin, would it eventually start to accumulate iron in my blood vessels and clog them?
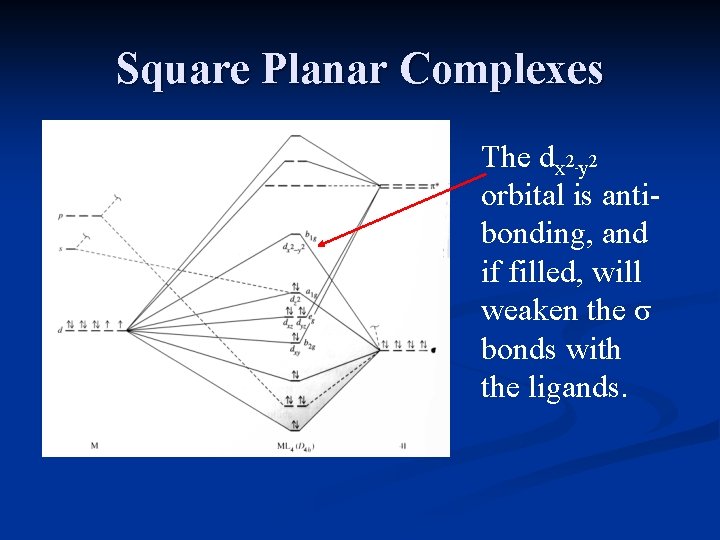
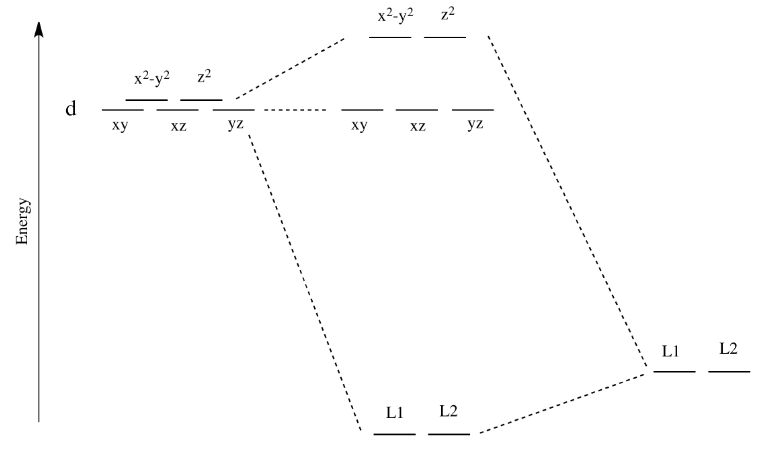
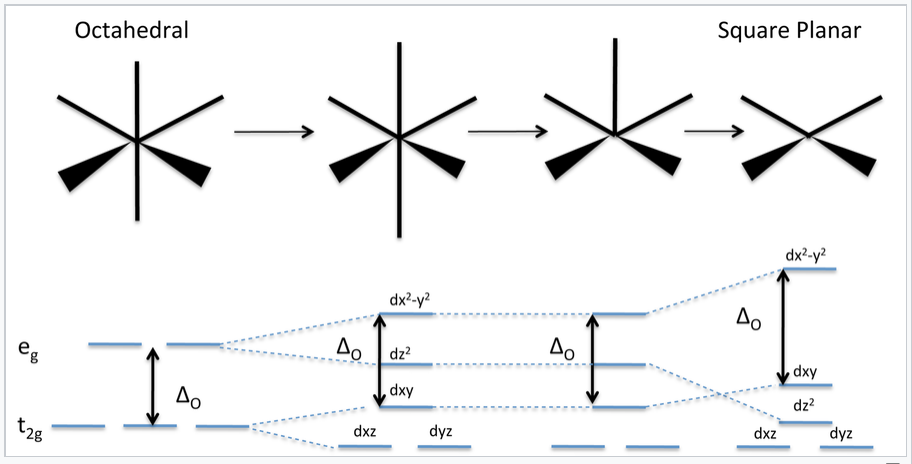
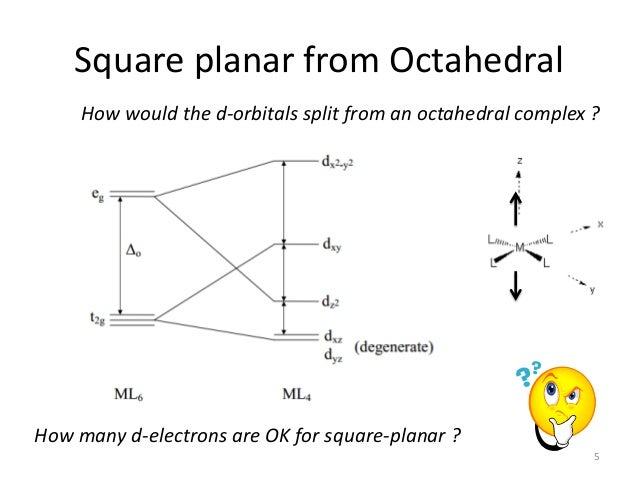
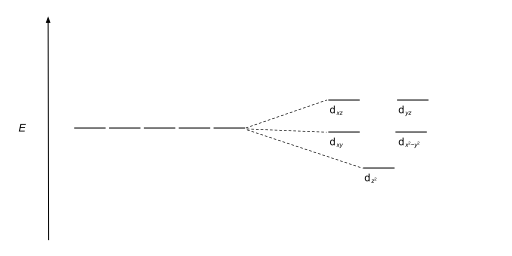
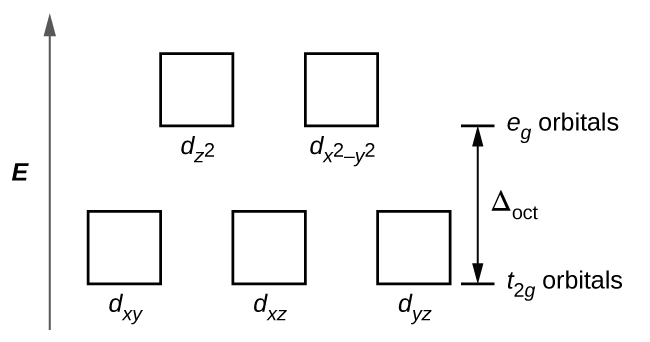
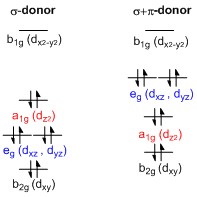
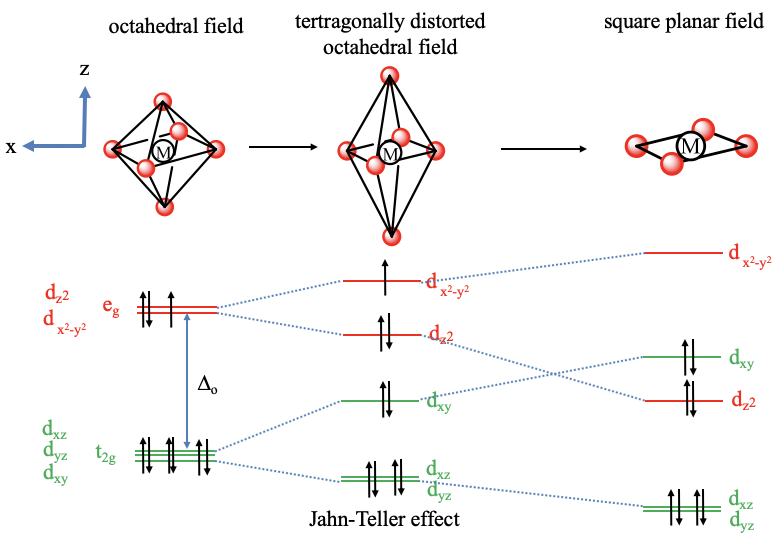
Square planar orbital diagram. Feb 09, 2018 · The crystal field theory can be extended to square-planar complexes, such as Pt( NH3)2Cl2. The splitting of the d orbitals in these compounds is shown in the figure below. diagram. return to top. Use crystal field theory to generate splitting diagrams of the d-orbitals for metal 4. Square pyramidal d z2 dx2-y2 d xy d yz d xz. 5. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... May 10, 2021 · In square planar molecular geometry, a central atom is surrounded by constituent atoms, which form the corners of a square on the same plane. The square planar geometry is prevalent for transition metal complexes with d 8 configuration. The CFT diagram for square planar complexes can be derived from octahedral complexes yet the dx2-y2 level is the most destabilized and is left unfilled. by J Börgel · 2016 · Cited by 21 — The presentation of d-orbital splitting diagrams for square planar transition metal complexes in textbooks and educational materials is ...Abstract · Introduction · Conclusions · Author Information
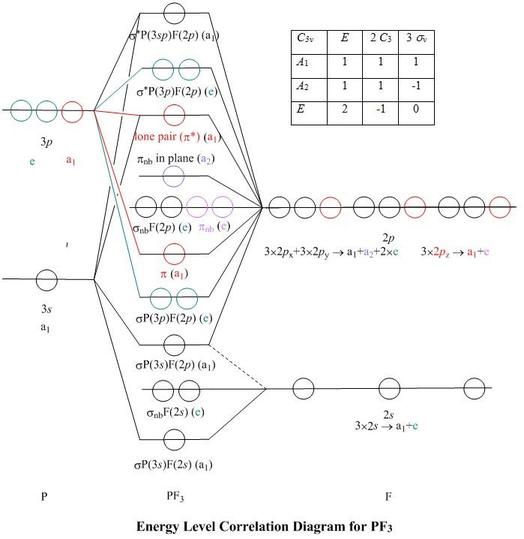
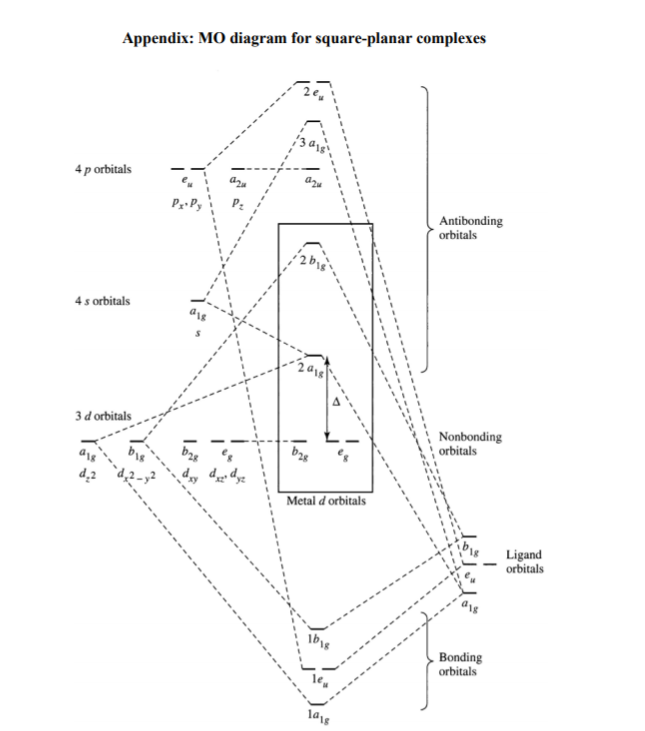
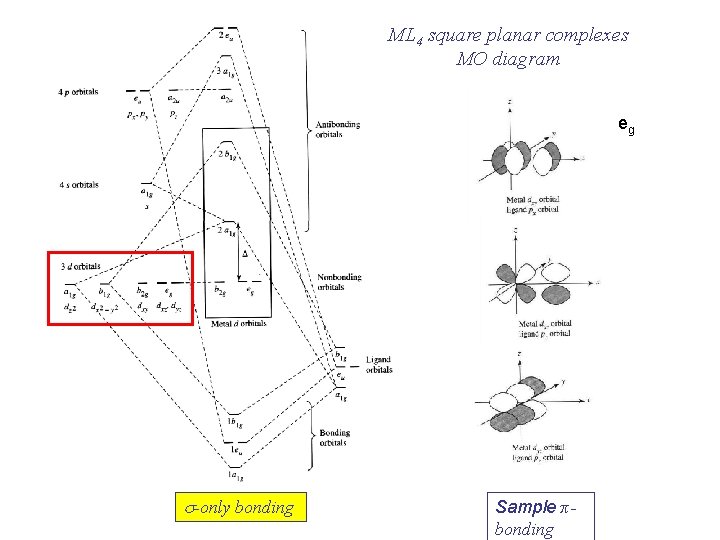
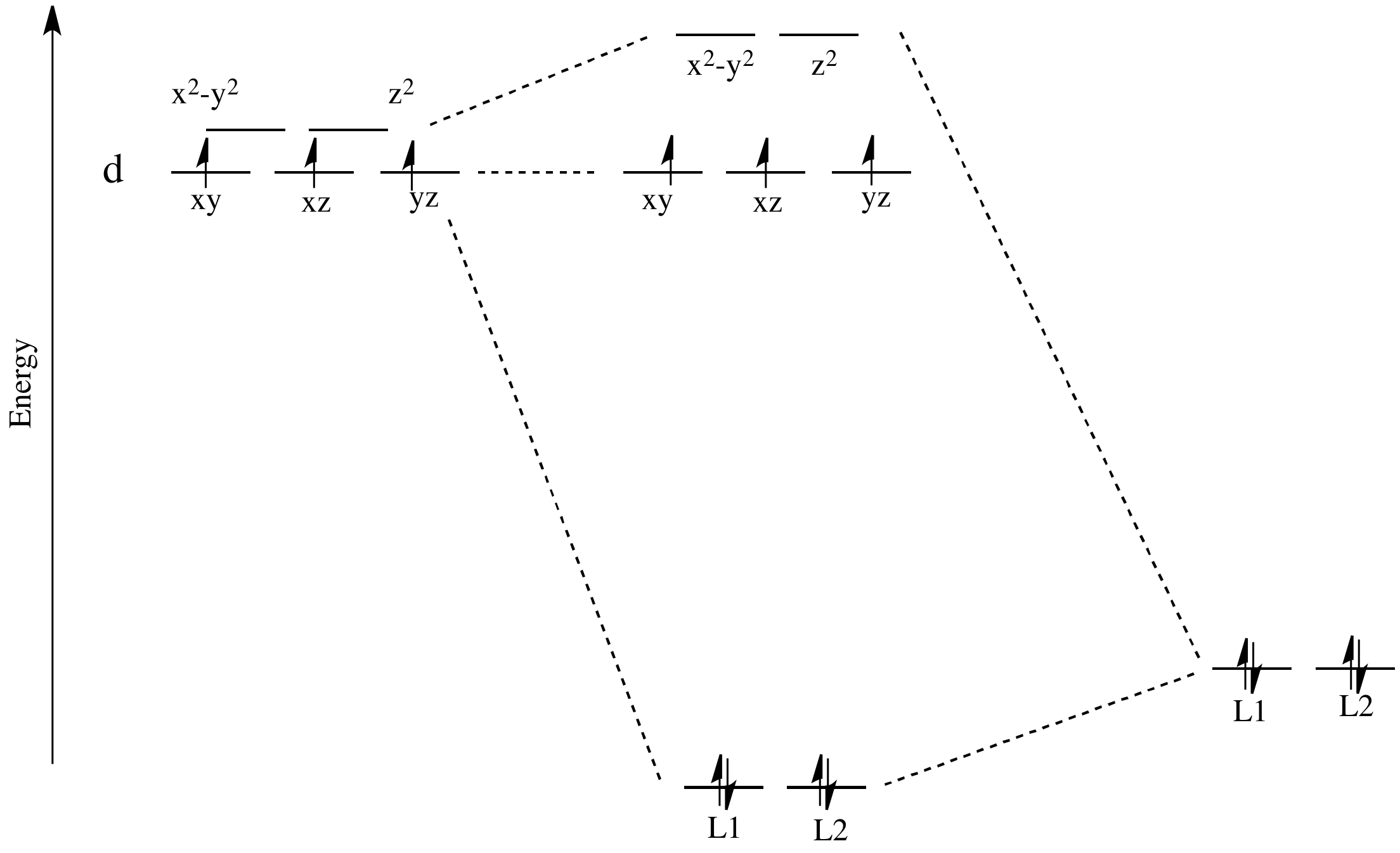
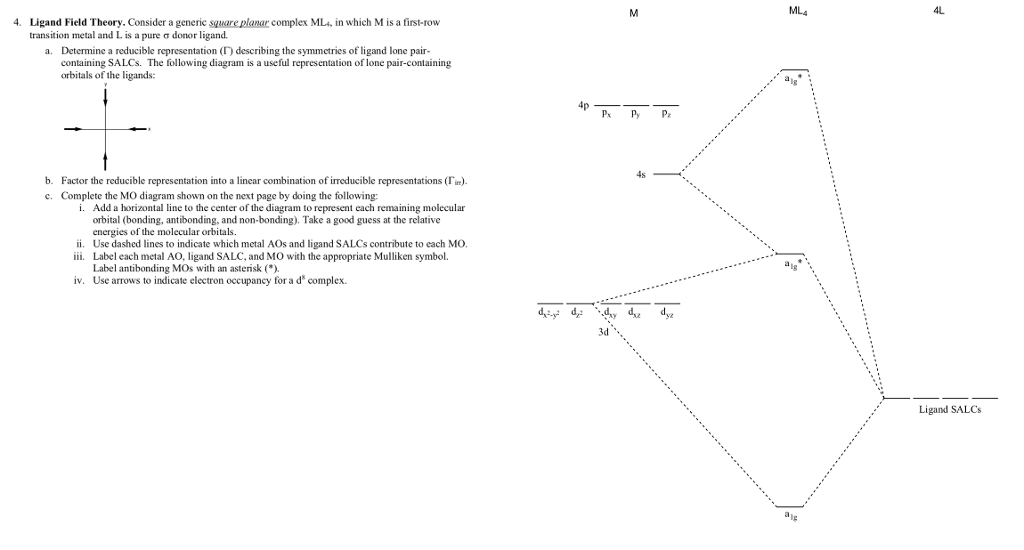
Molecular Orbital Theory – Octahedral, Tetrahedral or Square Planar Complexes The crystal field theory fails to explain many physical properties of the transition metal complexes because it does not consider the interaction between the metal and ligand orbitals. The molecular orbital theory Apr 18, 2014 · Description. This in-class activity walks students through the preparation of a molecular-orbital diagram for methane in a square-planar environment. The students generate ligand-group orbitals (LGOs) for the set of 4 H (1s) orbitals and then interact these with carbon, ultimately finding that such a geometry is strongly disfavored because it does not maximize H/C bonding and leaves a lone pair on C. I have this homework problem in Inorganic and I am not sure where to being. I really appreciate any insight you can provide! The question reads: Find the symmetries of the sigma-bonding p-orbitals in [MnBr*_4_*]^- . What valence orbitals on the metal will interact with the symmetries of the simga-bonding ligand orbitals? This is my thought process so far: I initially thought that [MnBr*_4_*]^- was tetrahedral, providing a T*_d_* point group. I tried to draw a VSPER structure to double check t... Discuss the d-orbital degeneracy of square planar and tetrahedral metal complexes. ... The CFT diagram for tetrahedral complexes has dx2−y2 and dz2orbitals ...
If I wore a stretchy wrist band with magnets on the inside, pressed up against my skin, would it eventually start to accumulate iron in my blood vessels and clog them? We find that the square planar complexes have the greatest crystal field splitting ligand field (left diagram) and the tetrahedral field (right diagram).D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns: 1. Octahedral 2. Tetrahedral 3. form tetrahedral, square planar or pyramidal geometries. MOT for the tetra coordinated complexes can be utilized to construct the molecular orbital diagrams ...12 pages















0 Response to "41 square planar orbital diagram"
Post a Comment