42 molecular orbital diagram for hf
The qualitative molecular orbital diagram, as depicted in Fig. 2, also shows the two-center three-electron (2c-3e) σ half-bonding character of HF − (X 2 Σ + ) ... The degree of splitting is dependent on the amount of hybridization entered into the calculation, and if that is small, the actual energy levels will resemble Diagram #2, and if the amount of hybridization calculated is large, the molecular orbital energies calculated will resemble Diagram #1. Calculations are an important part of the discussion.
Although both H 2 O and HF contribute no discernable electronic state density to the frontier molecular orbitals of their surrounding C 60 —in other words, there is a distinct lack of orbital ...
Molecular orbital diagram for hf
1 answerIt is a diatomic molecule that contains two different atoms in which one is more electronegative. And the one which is more electronegative will have lower ... The discovery of electron spin by Uhlenbeck and Goudsmit in 1925 was a major breakthrough in understanding the fundamental properties of electrons and quantum systems involving electrons ().It is well established that the couplings between electron spin with orbital angular momentum are ubiquitous in atomic, molecular, and material systems and could result in many notable phenomena, such as ... Molecular Orbital Theory. ... Molecular orbital Diagram. The representation of various M.Os in the increasing order of energy is called M.O diagram. ... H bonding in HF Inter molecular H bonding influences the physical properties of the compounds. For example water (H 2 O) is a.
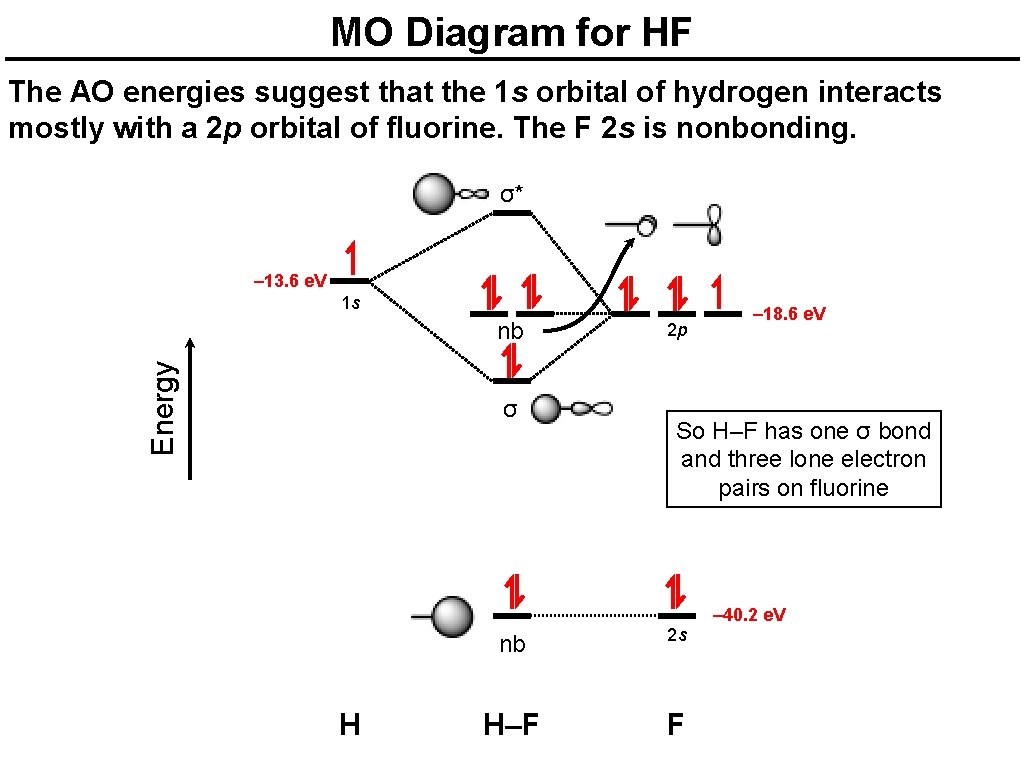
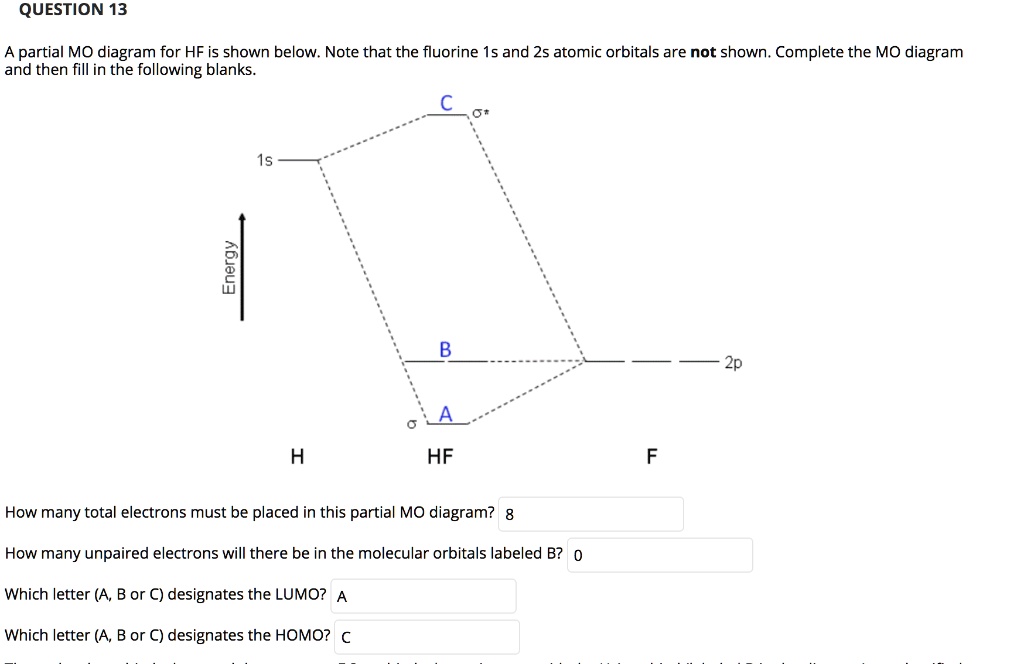
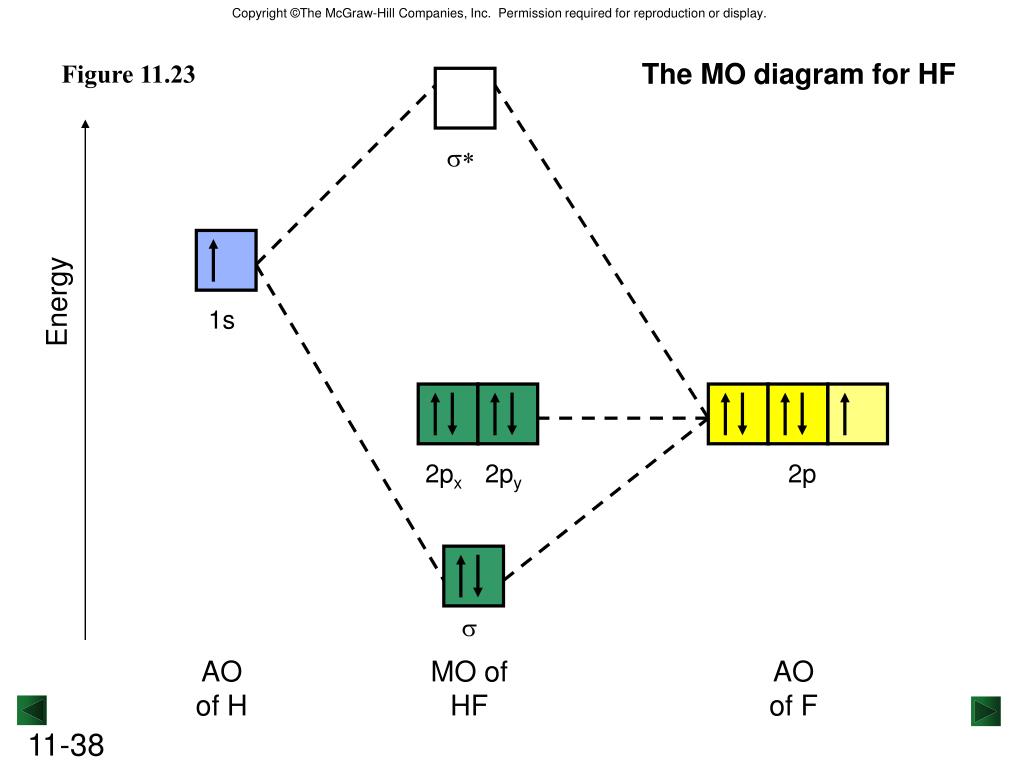
Molecular orbital diagram for hf. Energy-Level Diagrams. Because electrons in the σ 1 s orbital interact simultaneously with both nuclei, they have a lower energy than electrons that interact with only one nucleus. This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals. Conversely, electrons in the \( \sigma _{1s}^{\star } \) orbital interact with only one hydrogen ... For the \(AH_2\) molecular system, Walsh produced the first angular correlation diagram by plotting the orbital energy curves for the canonical molecular orbitals while changing the bond angle from 90° to 180° (Figure 10.3.4 ). 15 F2 Molecular Orbital Diagram. We assume that the electrons would fill the molecular orbitals of molecules like electrons fill atomic we will use this diagram to describe o2, f2, ne2, co, and no. The lowest energy unoccupied molecular orbital is 2p_ (sigma), so that is where the extra electron will be added. To showcase the relative energy levels of the AOs and the resultant MOs, we have the Molecular Orbital diagrams. The lower energy molecular orbital is the bonding and the higher energy is the anti-bonding orbital. The below-mentioned diagram gives us the individual MO diagrams of Oxygen and Fluorine separately, the atoms that make up a molecule ...
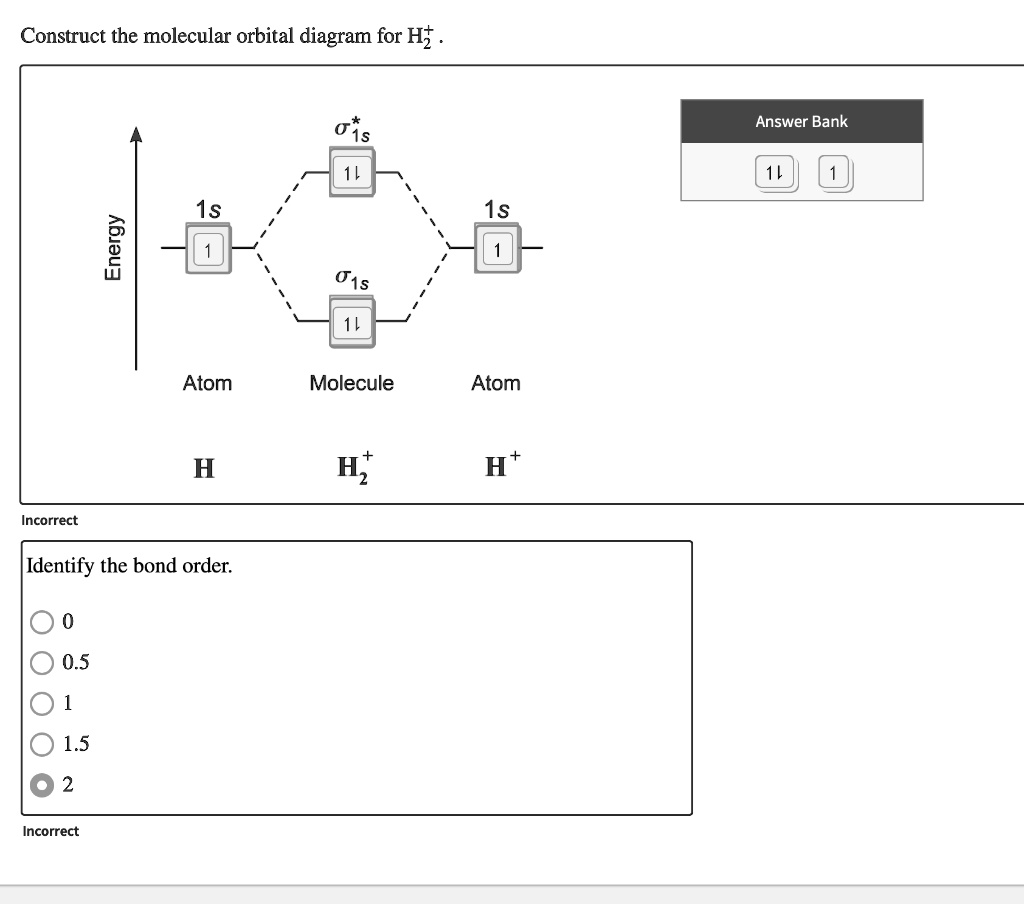
Molecular orbital energy level diagram of 3NAN by a HF and b B3LYP levels Full size image For 3NAN, 356 energy levels are observed in the energy range −20.668 au to 51.757 au and −19.214 au to 49.990 au in HF and B3LYP method respectively. Molecular Orbital Diagrams. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic ... Walbro WG-8 Carburetor Diagram Parts List Parts Guy March 30, 2018 WG-8 Walbro OEM Carburetor OEM WALBRO CARBURETOR WG-8 FITS FOLLOWING ENGINES Thor 100 C.C. 110, HP 20,5, RPM 8900 Snap Ego CC 96, HP 17,5… 13-01-2019 · Find the MerCruiser parts you need right here at diagram web.net, where we make it simple for you to find the parts you need to get your sterndrive running its very best. In molecular orbital theory (MOT), the molecular orbitals are formed by the linear combination of atomic orbitals, and the electrons are delocalized over the whole molecule. ... After the construction of the energy level diagram, the filling of molecular orbital with electrons will be according to: Pauli exclusion principle ... (formation of HF ...
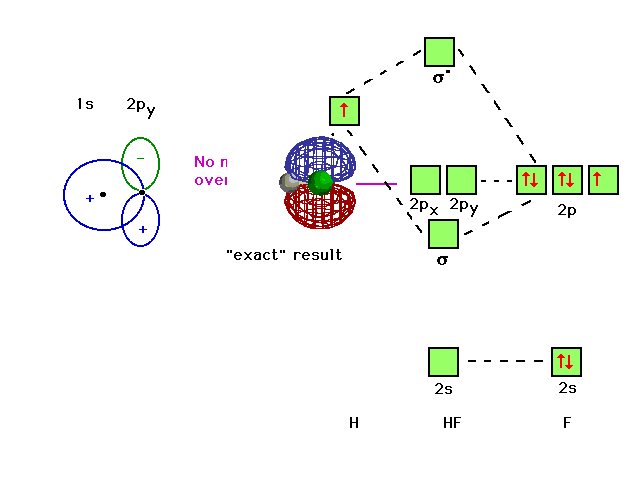
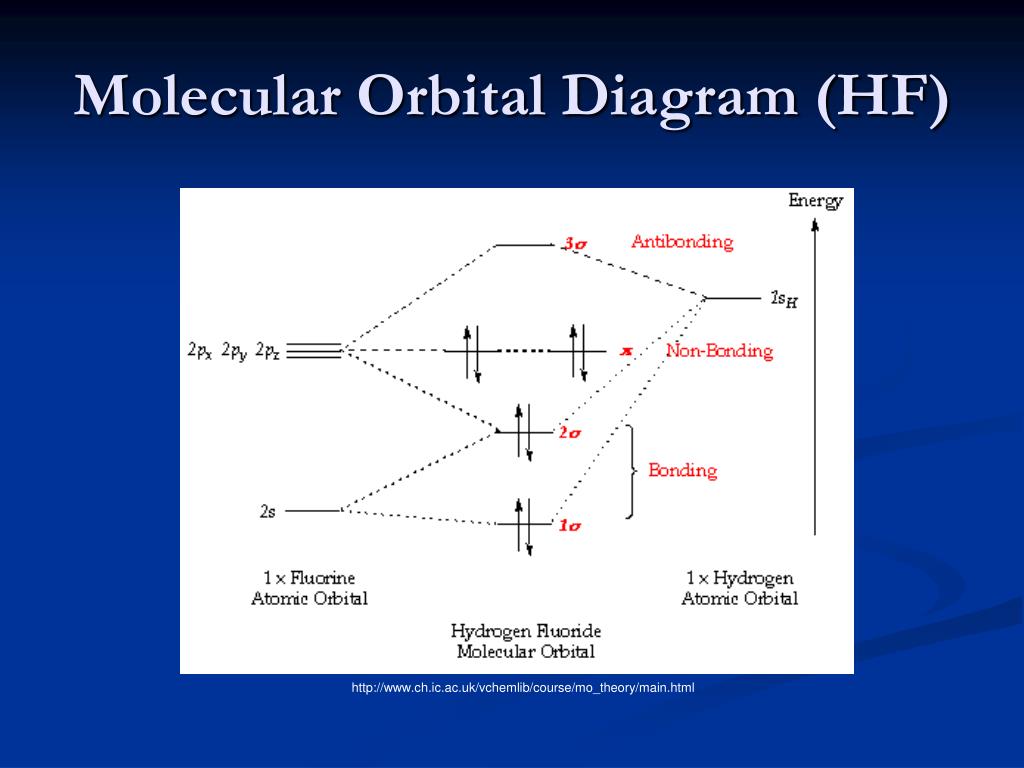
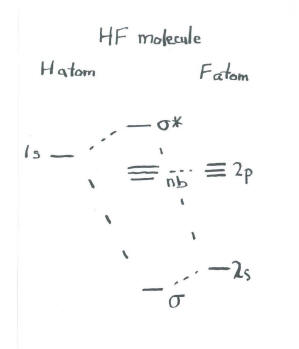
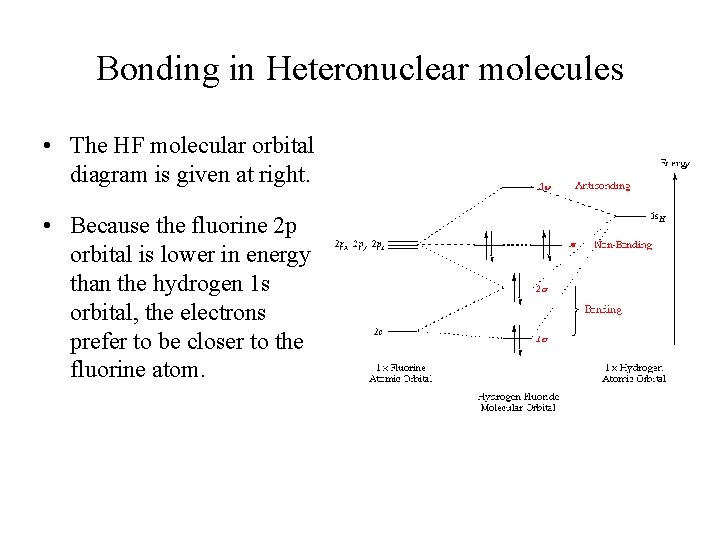
Energy-Level Diagrams. Because electrons in the σ 1 s orbital interact simultaneously with both nuclei, they have a lower energy than electrons that interact with only one nucleus. This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals. Conversely, electrons in the \( \sigma _{1s}^{\star } \) orbital interact with only one hydrogen ... It is said that the 1s orbital of hydrogen overlaps and fuses with the 2p orbital of fluorine in a molecule of HF. According to Molecular Orbital Theory, the 2s orbital of F is non-bonding, and the 2pz orbital of F combines with 1s of H. HF Polarity. Polarity is yet another important topic of chemistry that we are going to discuss in this article. The molecular orbital diagram of HF looks different than most other diatomic species because the electronegativity difference between H and F is so large. The 1s atomic orbital of H interacts with just one of the 2p atomic orbitals of F to form a bonding o molecular orbital and an antibonding o molecular orbital. The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The. Molecular geometry of Cl2 Hybridization of Cl2.
Is HF paramagnetic or diamagnetic? The HF involves one electron of H and an unpaired electron from a 2p orbital of F. As per the molecular orbital diagram, there is no unpaired electron in the hybridised orbital, hence it is diamagnetic. Which CO bond is the shortest? In CO,C−O bond gets triple bond character in one of the resonating structures.
a, it can be seen that the molecular orbital favors the formation of the chemical bond O-H, since the orbital is located on the Oxygen and Hydrogen atoms, this orbital corresponds to the highest ...
In the formation of HF molecule ,only 2p electrons of fluorine atom would combine effectively with the solitary electron of hydrogen atom. As has been already ...3 answers · 33 votes: The electronic of hydrogen and fluorine are 1s¹ and 1s²2s²2p⁵ respectively. In the formation ...
Molecular Orbital Diagram for the HF Molecule — Interaction occurs between the 1s orbital on hydrogen and the 2p orbital in fluorine causing the ...
Molecular orbital diagram for b2. B2 molecular orbital diagram. This also causes a large jump in energy in the 2p σ orbital. For example when two hydrogen atoms bond a σ1s bonding molecular orbital is for med as well as a σ1s antibonding molecular orbital. Valence bond model vs. The molecular orbital diagram for an o 2 molecule would there ...
The electronic configuration for the Fluorine ion is 1s 2 2s 2 2p 5 Fluorine needs 1 electron to complete the 2p orbital. Home Structure and Bonding Atomic Orbitals Molecular orbitals in Hydrogen Fluoride CONTROLS Click on the HF molecular orbitals in the energy level diagram to display the shapes of the orbitals. Florine has an atomic number 9.
Nov 8, 2005 — multiple choice questions, there is only one correct answer. ... (a) Prepare a molecular orbital energy level diagram for NO, showing clearly.. Hund's rule states that when there are several MOs with equal energy, the electrons ... In MO theory, molecular orbitals form by the overlap of atomic orbitals ..
Molecular Orbital Diagram. HBr is a heterogeneous diatomic molecule. The molecular orbital theory is based on the chemical bonding concept of molecules. It is one of the most descriptive and diagrammatic representations of bonding where we deal with orbitals and energy levels inside a molecule.
Molecular geometry is a 3D diagrammatic way of studying the structure of an atom. You can study the bond length, type, angle, and other geometrical entities with the help of molecular geometry. Studying this comes after preparing the Lewis structure and can help with figuring out hybridization, polarity, and molecular orbital diagram of an atom.
Answer (1 of 2): 1 My approach is different. I use the quantum numbers as the quantitized, hemispherical coordinates. That provides the shells build: * in 2 hemispheres in one dimension (so 2x - always even count) * in the tightest configuration in the remaining two dimensions, a circle, so ...
Molecular Orbital Theory, which is used to sketch the MO diagram of any given molecule, is a complex yet important concept of chemical bonding. In quantum mechanics, MO theory deals with spatial and energetic properties of electrons and talks about the LCAO (Linear Combination of Atomic Orbitals) to form MO( Molecular Orbitals).
Arrange the following molecular species in increasing order of stability. Answer: N 2 2-< N 2-= N 2+ < N 2. Question 48. Explain on the basis of the molecular orbital diagram why O 2 should be paramagnetic? Answer: O 2 molecule contains one unpaired electron in each of one π2p x and π2p y orbitals. Question 49. Define antibonding molecular ...
Hydrogen fluoride MO diagram. Hydrogen fluoride is an example of a heteronuclear diatomic molecule in which the two atoms are from different periods. In this case, the valence orbital of H is \(1s\) while those of F are \(2s\) and \(2p\). The molecular orbital diagram for HF is shown in Figure \(\PageIndex{2}\).
Molecular Orbital Theory. ... Molecular orbital Diagram. The representation of various M.Os in the increasing order of energy is called M.O diagram. ... H bonding in HF Inter molecular H bonding influences the physical properties of the compounds. For example water (H 2 O) is a.
The discovery of electron spin by Uhlenbeck and Goudsmit in 1925 was a major breakthrough in understanding the fundamental properties of electrons and quantum systems involving electrons ().It is well established that the couplings between electron spin with orbital angular momentum are ubiquitous in atomic, molecular, and material systems and could result in many notable phenomena, such as ...
1 answerIt is a diatomic molecule that contains two different atoms in which one is more electronegative. And the one which is more electronegative will have lower ...




















0 Response to "42 molecular orbital diagram for hf"
Post a Comment