43 ozone molecular orbital diagram
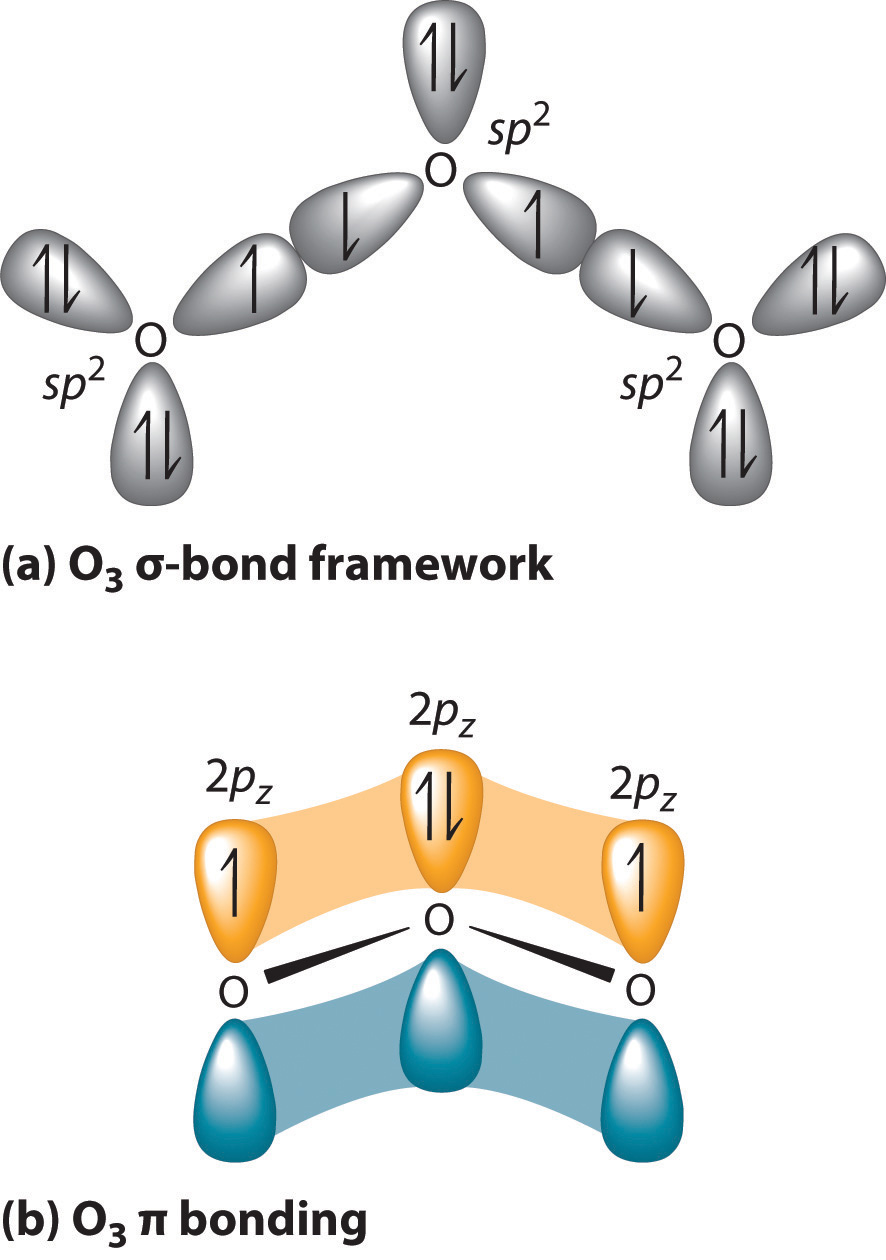
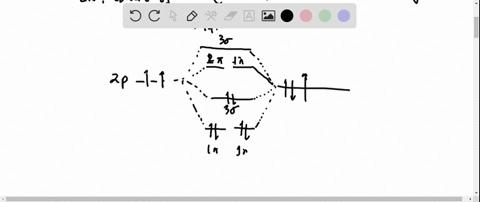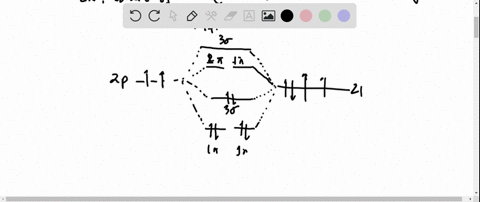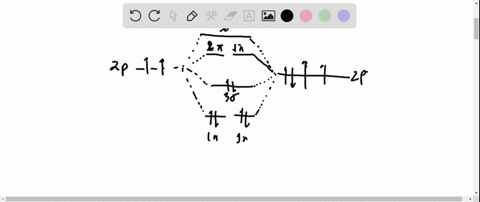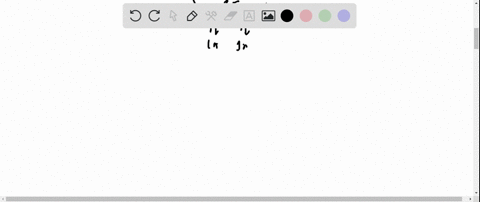
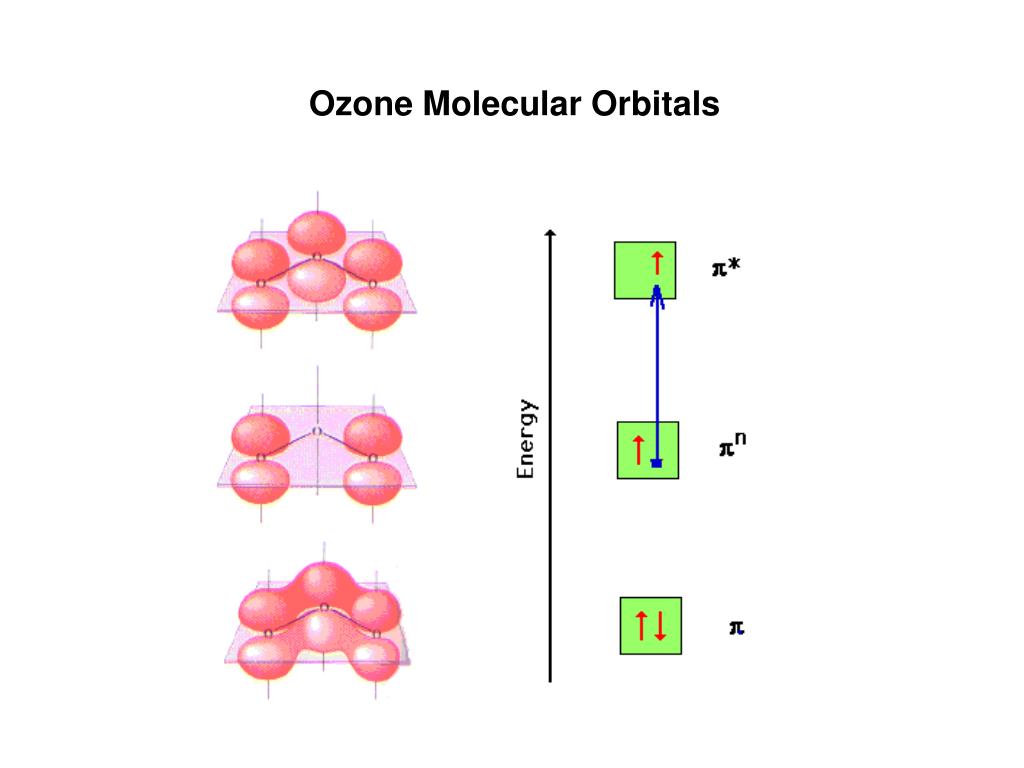
Ozone Let's look at the molecular orbital diagram of ozone. We'll use the hybrid orbital approximation. Each oxygen atom combines its 2s, 2p z and 2p y orbitals to make three 2sp2 hybrid orbitals. • O1 uses one 2sp 2 orbital to combine with one 2p orbital of O2, making a sigma bonding and sigma antibonding orbital Conjugated System is a molecular entity whose structure can be represented as a system of alternating single and multiple bonds; Ozone Depletion. Ozone is a bent molecule with bond angle 116.8° It has a resonance structure; UV light radiation from the sun is very high in energy and is able to break the oxygen to oxygen bonds in ozone.
Printable O2 molecular orbital diagrams are available for you to guide your study in the molecular orbital lesson.This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
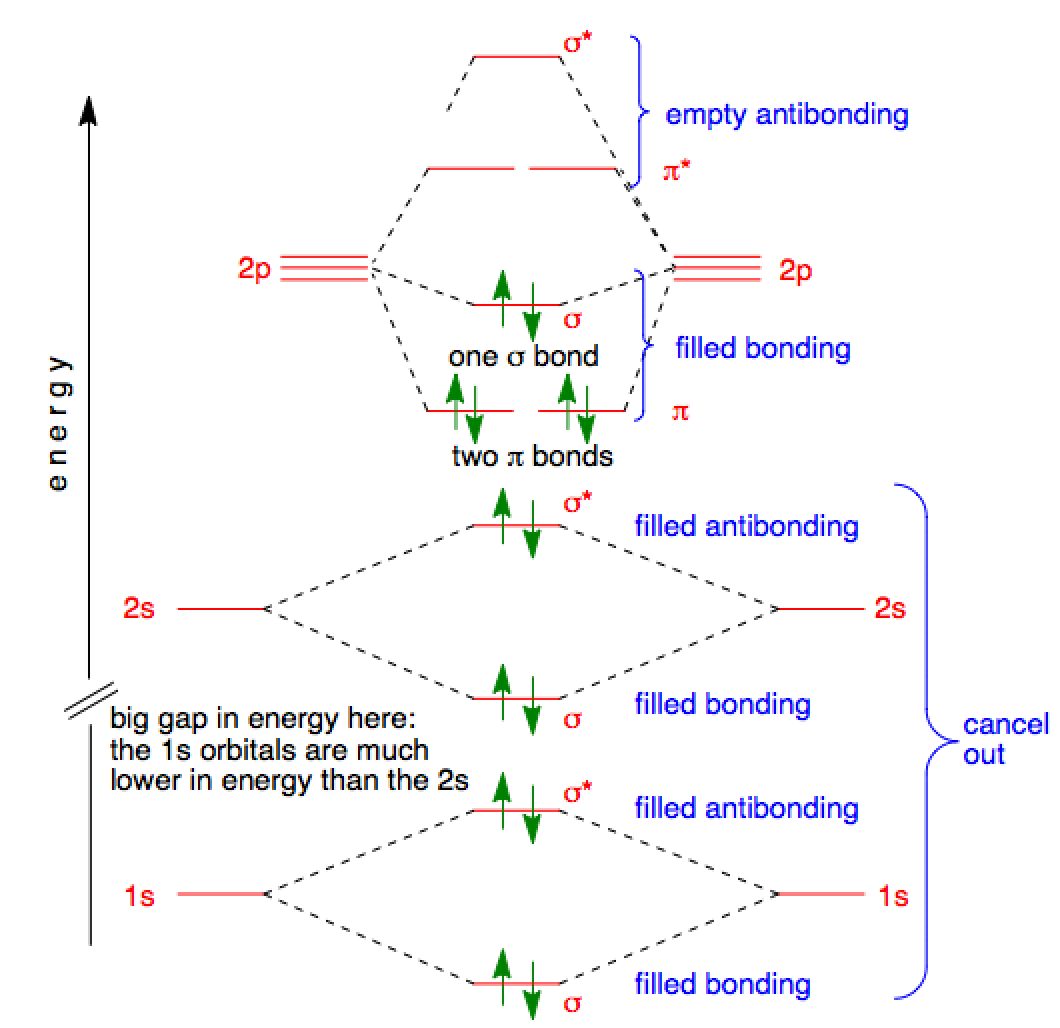
Ozone molecular orbital diagram
Draw a molecular orbital diagram for ozone. August 29, 2020 / in / by developer. Draw a molecular orbital diagram for ozone. Show the reducible(if applicable) and irreducible representations for each set of group orbitals constructed and the symmetry of each orbital or group orbitals used in the diagram by TY Takeshita · 2015 · Cited by 26 — O3 and SO2 using both molecular orbital (MO) and valence ... represent the GVB wave function using simple orbital diagrams. Molecular Orbitals. MO14. Delocalized Bonding: Conjugation in Ozone. Ozone is a fairly simple molecule, with only three atoms. However, in order to focus on one aspect of ozone's structure, we will use a hybrid approximation in order to simplify the picture. The Lewis structure of ozone is somewhat unsatisfactory.
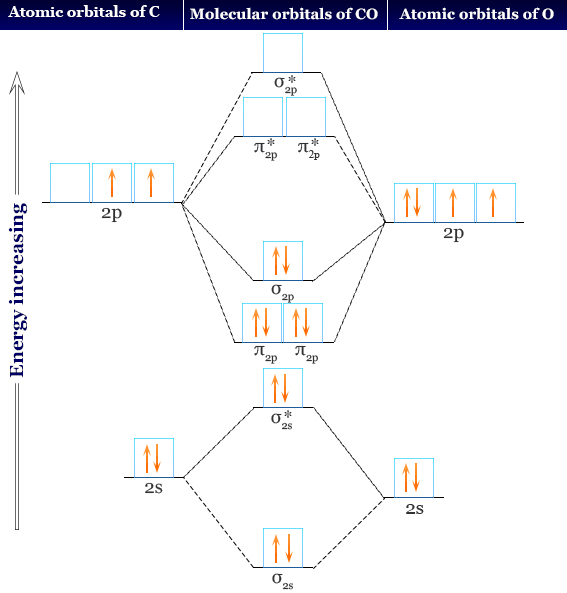
Ozone molecular orbital diagram. A molecular orbital diagram of ozone is at right, but it's not terribly useful in its current form because it doesn't include the electrons. Recall that bonding is a valence-electron phenomenon, that only valence orbitals contribute to a molecular orbital diagram, and that electrons generally fill non-degenerate orbitals two at a time. 2b1 a) The molecular orbital diagram representing this order of energy levels is shown in fig. Fig. No. 5 Order of Energy Levels for Boron, Carbon, Nitrogen etc. This kind of energy reversal is due to mixing of 2s and 2p orbitals where the energy difference is very close, that is, for B, C, and N atoms. According to the symmetry interactions, the two ... The total energy of the electrons in the molecular orbitals is ... The MO diagram of CO helps explain its reaction chemistry with transition metals, which.52 pages O3 Molecular Orbital Diagram (MO) The molecular orbital theory is one of the major revolutionary concepts of chemical bonding. It uses quantum mechanics to give us a detailed almost explanatory diagram of the bonding nature inside a molecule. Here is a diagrammatic representation of the MO diagram of ozone.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... Brief tutorial on how ozone bonds. Molecular orbitals and organic chemical reactions/Ian Fleming. — Reference ed. p. cm. Includes bibliographical references and index. ISBN 978--470-74658-5 1. Molecular orbitals. 2. Chemical bonds. 3. Physical organic chemistry. I. Title. QD461.F533 2010 5470.2—dc22 2009041770 A catalogue record for this book is available from the British ... O3 Molecular Orbital Diagram (MO) ... The molecular orbital theory is one of the major revolutionary concepts of chemical bonding. It uses quantum mechanics to ...
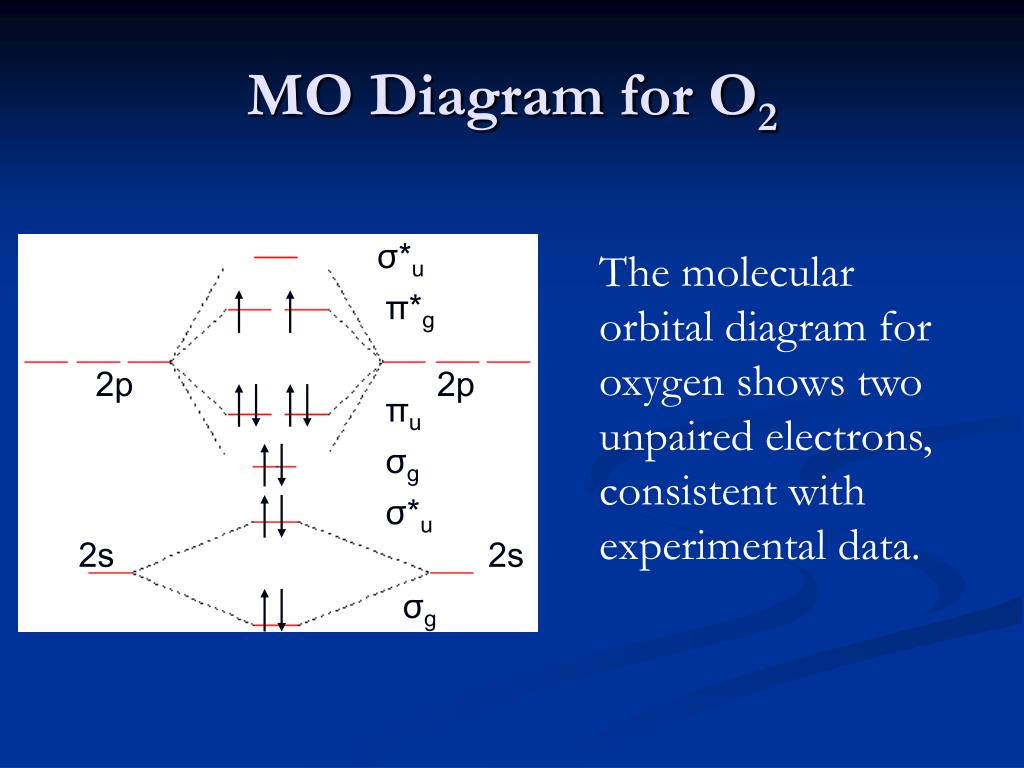
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Ozone is an elemental molecule with formula O3. An explosive, pale blue gas (b.p. -112℃) that has a characteristic, pleasant odour, it is continuously produced in the upper atmosphere by the action of solar ultraviolet radiation on atmospheric oxygen.It is an antimicrobial agent used in the production of bottled water, as well as in the treatment of meat, poultry and other foodstuffs. In the table of ozone molecular orbit. on one atom. These diagrams show the difference between valence bond theory and molecular orbital theory when considering the orbitals of benzene. C C. C. OF- has 14 valence electrons, four in the π2p* orbitals (see the diagram in the .. Introduction to Inorganic Chemistry/Molecular Orbital Theory. The ... The molecule 1,3,5-hexatriene contains six p orbitals which all overlap but in a linear fashion. As with benzene, this overlap creates 3 stabilized bonding molecular which are completely filled with six p electron. As expected, the conjugation creates a marked increase of stability in 1,3,5-hexatriene but not as much as in benzene.
It may interact with the. (px, py) and (dxz, dyz) pairs of Ta. 5.19 The energy level diagram for O3 with the simple combinations of s and p orbitals is shown ...29 pages
Ozone (O3) is a highly reactive gas composed of three oxygen atoms. O3 Lewis structure comprises two oxygen atoms connected with one double bond and one single bond. The phrase "ozone layer" refers to the high concentration of ozone found in the stratosphere about 15-30 km above the earth's surface. It covers the whole globe and preserves life by absorbing the sun's damaging ...
Schematic molecular orbital diagram of O 3 and O ϩ 3 illustrating the ... molecular orbitals of ozone relevant for the interpre- tation of the photoelectron spectrum are depicted in Fig. 1. Early ...
Ozone is one of the most common examples used to study the Lewis structure. The molecule of Ozone has three oxygen atoms. It is written as O3 in the core chemistry equations. To understand the hybridization, polarity and molecular geometry of the Ozone molecule it is crucial to know the Lewis structure of the same. Name of molecule.
Molecular orbitals in Nitrogen. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. There are four molecular orbitals derived from the 1s and 2s orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The p orbitals combine to produce a sigma and two ...
Let's look at the molecular orbital diagram of ozone. We'll use the hybrid orbital approximation. Each oxygen atom combines its 2s, 2pz and 2py orbitals to make ...
Please draw MO diagram for ozone (O3). I saw a pic for O3 where the 2s of O was interacting with both bonding and antibonding. Can you please draw and it. Let's look at the molecular orbital diagram of ozone. We'll use the hybrid orbital approximation. Each oxygen atom combines its 2s, 2pz and 2py orbitals to make .
All molecular orbitals except the highest would be occupied by molecular orbitals in the diagram .. and 10 in the ozone diagram in the Problem answer. The ozone molecule's Lewis structure shows that even the preferred structure The pi molecular orbital energy diagram for ozone into which are distributed four .
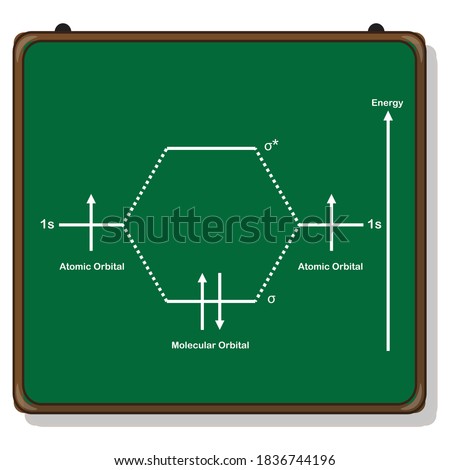
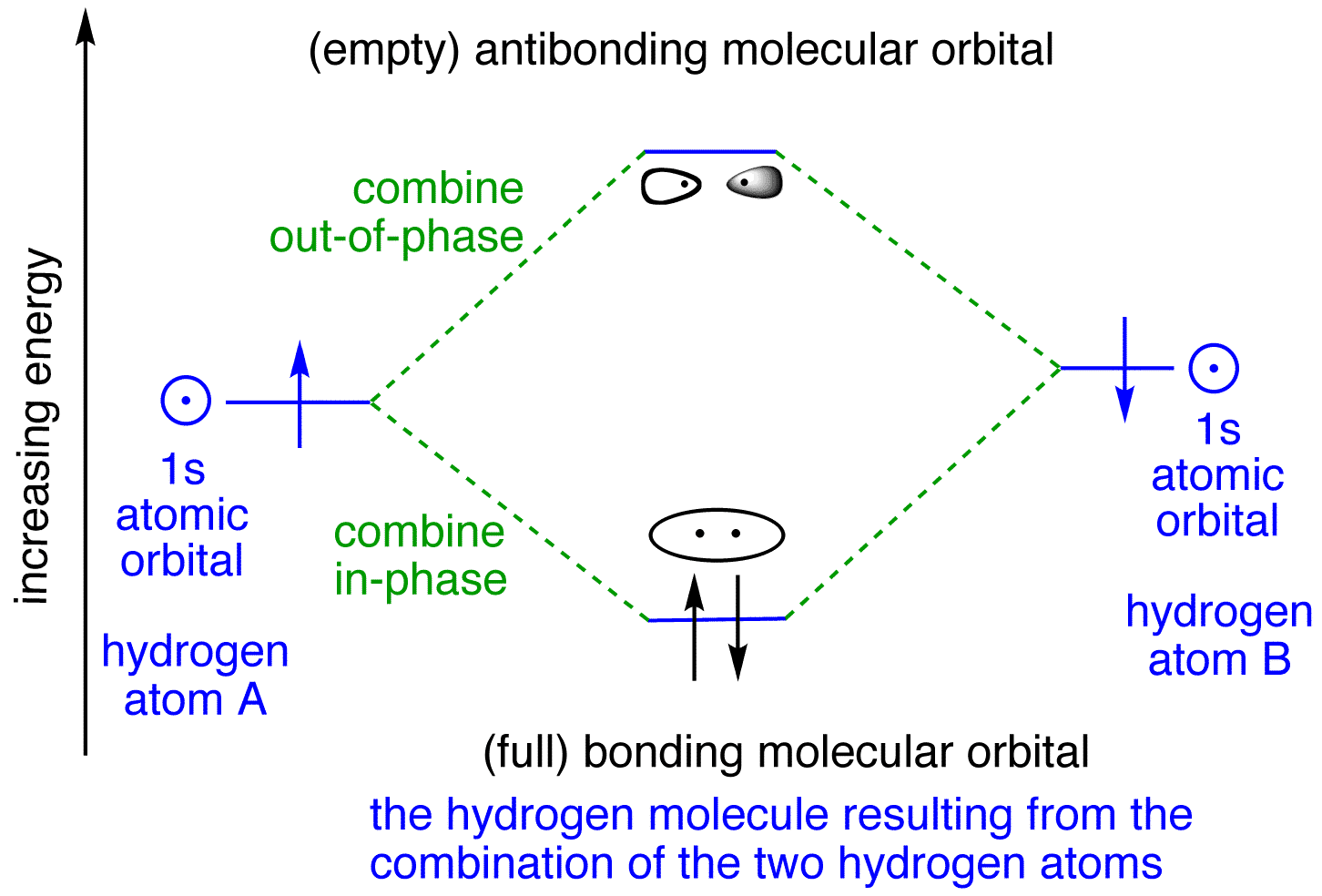
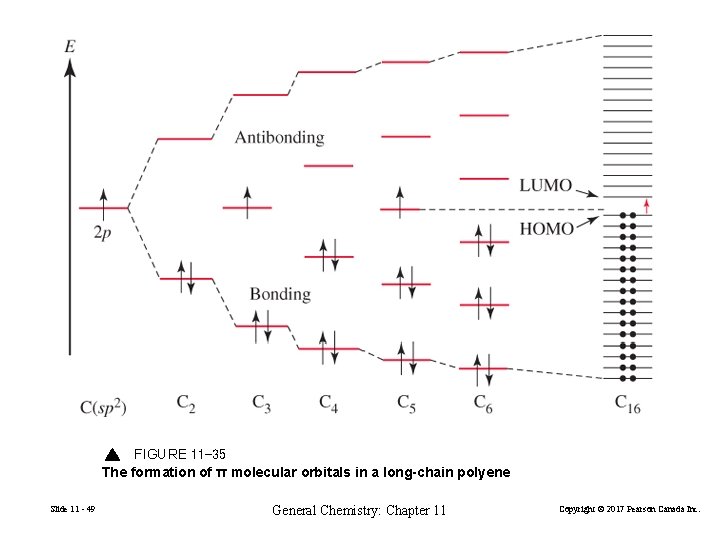
In general, this mixing of n atomic orbitals always generates n molecular orbitals. The hydrogen molecule provides a simple example of MO formation. In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher energy MO referred to as an antibonding orbital.
The localized molecular orbital theory and energy partitioning formalism have been invoked to study the structure and bonding in ozone molecule. The range of investigation covers a large number of ...
May 8, 2021 — Molecular Orbitals and Resonance Structures · Experimental evidence indicates that ozone has a bond angle of 117.5°. · With a molecular orbital ...
The pi molecular orbital energy diagram for ozone into which are distributed four valence electrons. Two are in the bonding orbital and yield a bond order ...
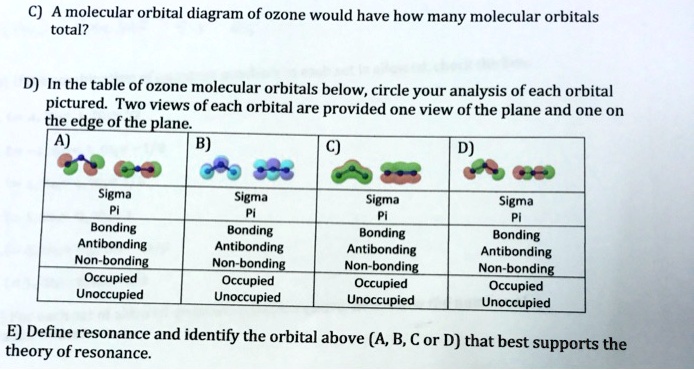
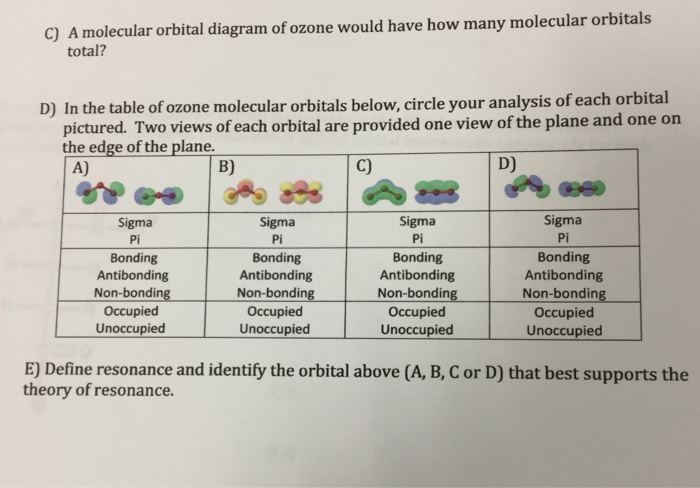
A molecular orbital diagram of ozone would have how many molecular orbitals total? In the table of ozone molecular orbitals below, circle your analysis of each orbital pictured. Two views of each orbital are provided one view of the plane and one on the edge of the plane. Define resonance and identify the orbital above (A, B, C or D) that best ...
(b) As in ozone, these orbitals can interact, in this case to form four molecular orbitals. The molecular orbital at lowest energy is a bonding orbital with 0 nodes, the one at highest energy is antibonding with 3 nodes, and the two in the middle have 1 node and 2 nodes and are somewhere between bonding or antibonding and nonbonding, respectively.
The molecular structure has been optimized at the B3LYP/6-31g* level of theory. Charges used for electrostatic maps are computed using the NBO method. The molecular vibrations are
Molecular Orbitals for Ozone Purpose: In this exercise you will do semi-empirical molecular orbital calculations on ozone with the goal of understanding the molecular orbital print out provided by Spartan and MOPAC at the MNDO level. The term semi-empirical means that some of the integrals necessary in the
Figure Molecular Orbital Energy-Level Diagram for \ (\pi\) Each oxygen atom in ozone has 6 valence electrons, so O 3 has a total of Let's look at the molecular orbital diagram of ozone. We'll use the hybrid orbital approximation. Each oxygen atom combines its 2s, 2pz and 2py orbitals to make .Colby College Molecular Orbitals for Ozone Purpose ...
Pi Bonds over 3 Atoms Ozone Let's look at the molecular orbital diagram of ozone. We'll use the hybrid orbital approximation. Each oxygen atom combines its 2s, 2p z and 2p y orbitals to make three 2sp 2 hybrid orbitals.. O 1 uses one 2sp 2 orbital to combine with one 2p 2 orbital of O 2, making a sigma bonding and sigma antibonding orbital; O 3 uses one 2sp 2 orbital to combine with a second ...
Molecular Orbitals. MO14. Delocalized Bonding: Conjugation in Ozone. Ozone is a fairly simple molecule, with only three atoms. However, in order to focus on one aspect of ozone's structure, we will use a hybrid approximation in order to simplify the picture. The Lewis structure of ozone is somewhat unsatisfactory.
by TY Takeshita · 2015 · Cited by 26 — O3 and SO2 using both molecular orbital (MO) and valence ... represent the GVB wave function using simple orbital diagrams.
Draw a molecular orbital diagram for ozone. August 29, 2020 / in / by developer. Draw a molecular orbital diagram for ozone. Show the reducible(if applicable) and irreducible representations for each set of group orbitals constructed and the symmetry of each orbital or group orbitals used in the diagram



















0 Response to "43 ozone molecular orbital diagram"
Post a Comment