44 carbon electron distribution diagram
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s and 2p subshells are occupied by 2, 2 and 6 electrons respectively. ...
22 Oct 2020 · 1 answerThe formation of Carbon dioxide (CO2) molecule.
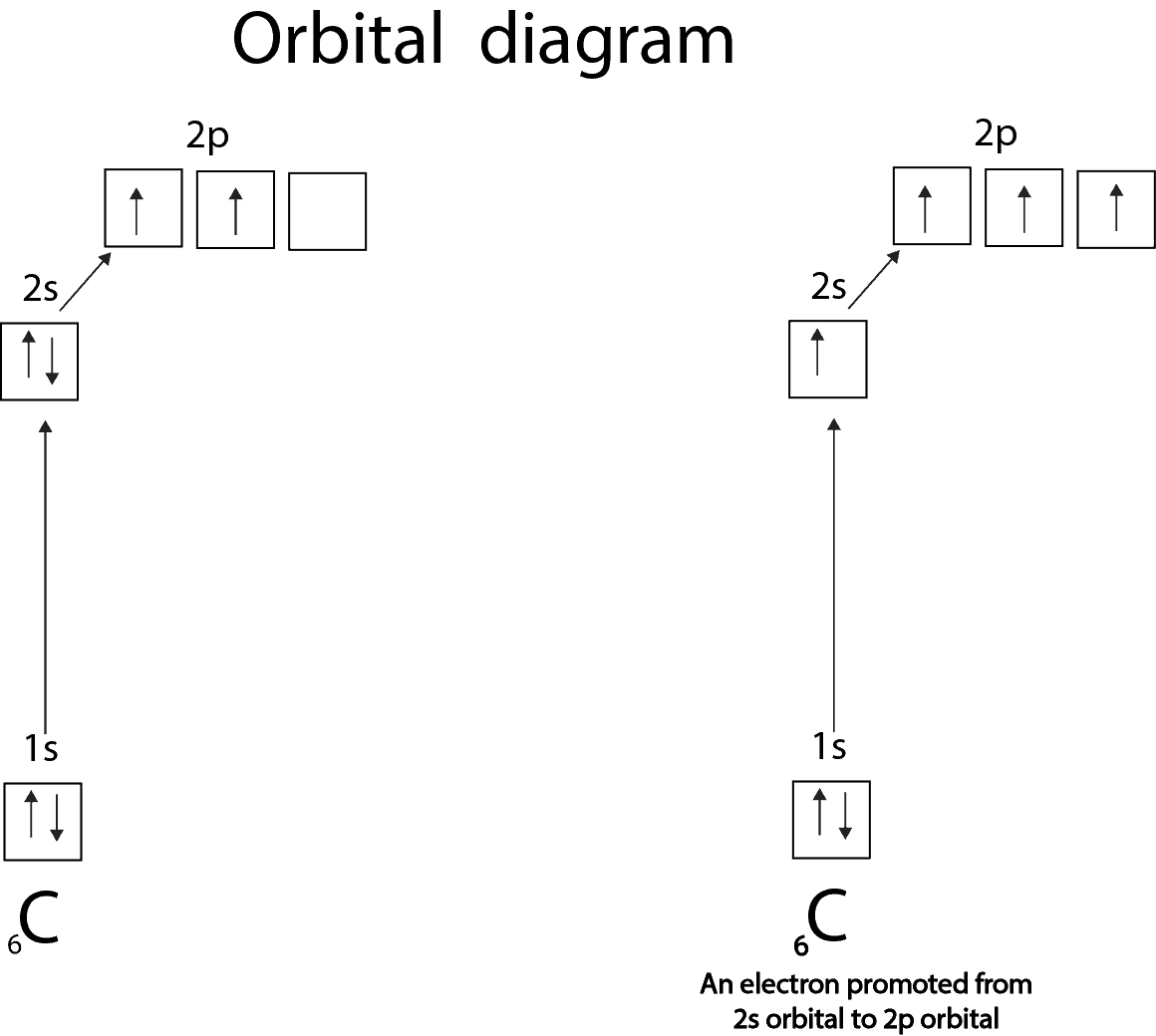
The electron configuration of carbon in excited state is C* (6) = 1s 2 2s 2 2p x1 2p y1 . Here, the electron configuration of carbon (C) shows that two unpaired electrons exist. In this case, the valency of the carbon atom is 2. When the carbon atom is excited more than this, the electron configuration of carbon changes again.
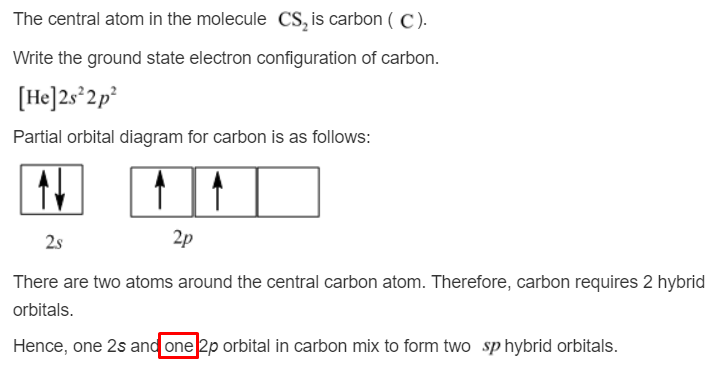
Carbon electron distribution diagram
The element carbon has 6 electrons in total and one of the main things that many users might not know is the symbol by which it is represented. The symbol of carbon is written as 6. Carbon Electron Dot Diagram. If we talked about the electronic configuration of the element then, carbon is an element whose electronic configuration is given as 1s ...
The total valence electron is available for drawing the carbon tetrabromide ( CBr4) lewis structure is 32. The hybridization of CBr4 is Sp 3 and the bond angle of 109.5°. CBr4 is a nonpolar molecule because of the zero net dipole moment caused by its symmetrical structure. In the CBr4 lewis structure, a total of 12 unshared and 4 shared pairs ...
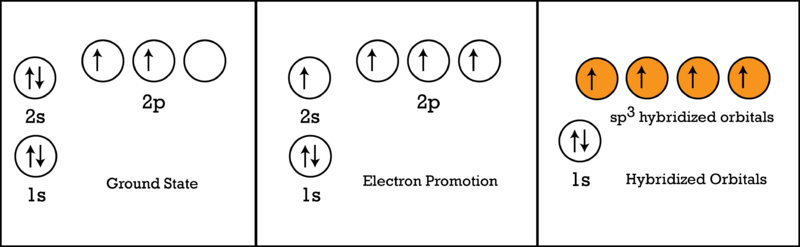
We illustrate the orbitals and electron distribution in an isolated carbon atom and in the bonded atom in CH 4 in Figure 11. The four valence electrons of the carbon atom are distributed equally in the hybrid orbitals, and each carbon electron pairs with a hydrogen electron when the C-H bonds form. Figure 11.
Carbon electron distribution diagram.
Make an electron distribution diagram of carbon it is essential posted on september 6 2018 by admin a diagram is shown in two parts connected by right facing arrow labeled zoom a polar bond is type of covalent chemical. Hydrocarbons are hydrophobic molecules consisting of only carbon and hydrogen such as benzene methane petroleum and fats.
Electron distribution diagram of carbon. C 12 c 13 and c 14 are isotopes of the element carbon. Greg robsoncc by 20. Sodium greg robsoncc by 20. The electron shells are shown moving outward from the nucleus. In atomic physics and quantum chemistry the electron configuration is the distribution of electrons of an atom or molecule or other physical structure in atomic or molecular orbitals. How ...
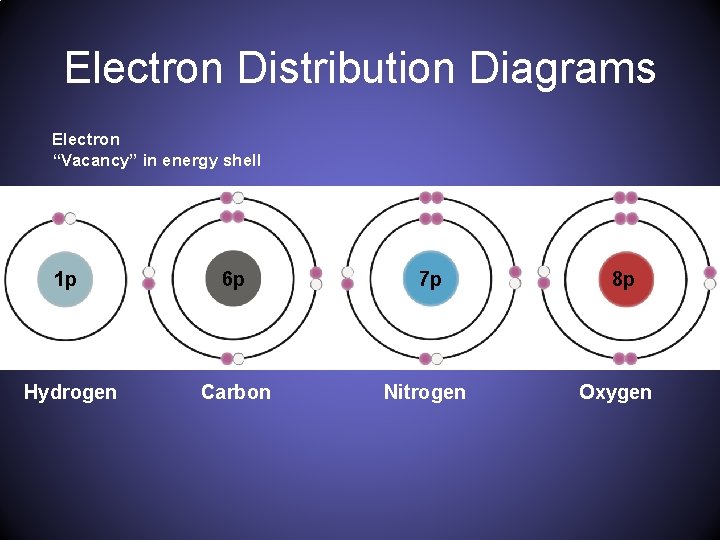
Electron Distribution Diagram. Hydrogen. 1. Carbon. 6. Oxygen. 8. Nitrogen. 7. Chlorine. 17. Sulfur. 16. Phosphorus. 15. 2. Name two trace elements, and list one specific function in human cells for each of these. a. Element: Function: b. Element: Function: 3. Draw a Bohr (electron distribution) diagram for the following two isotopes of sulfur: S-32 b. S-35. 4. Build a model of propane (C3H8 ...
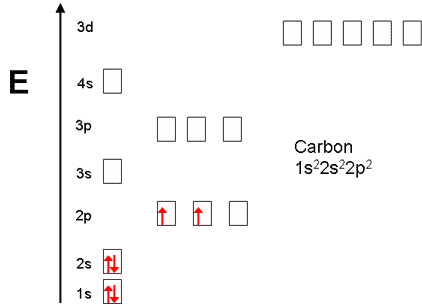
The electron configuration and orbital diagram for carbon are: Nitrogen (atomic number 7) fills the 1 s and 2 s subshells and has one electron in each of the three 2 p orbitals, in accordance with Hund's rule. These three electrons have unpaired spins.
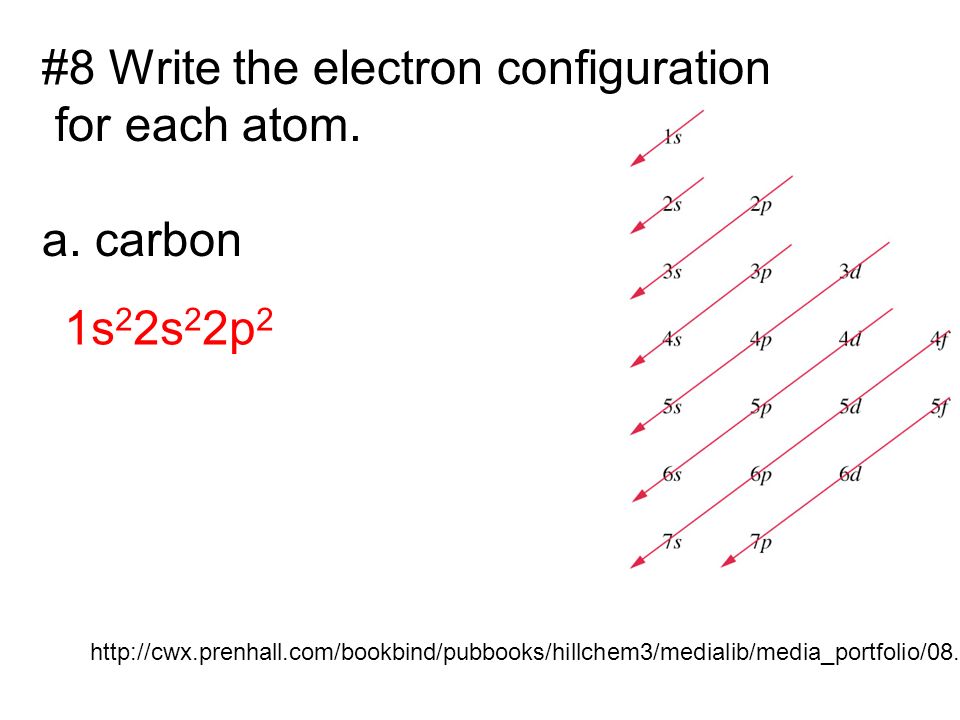
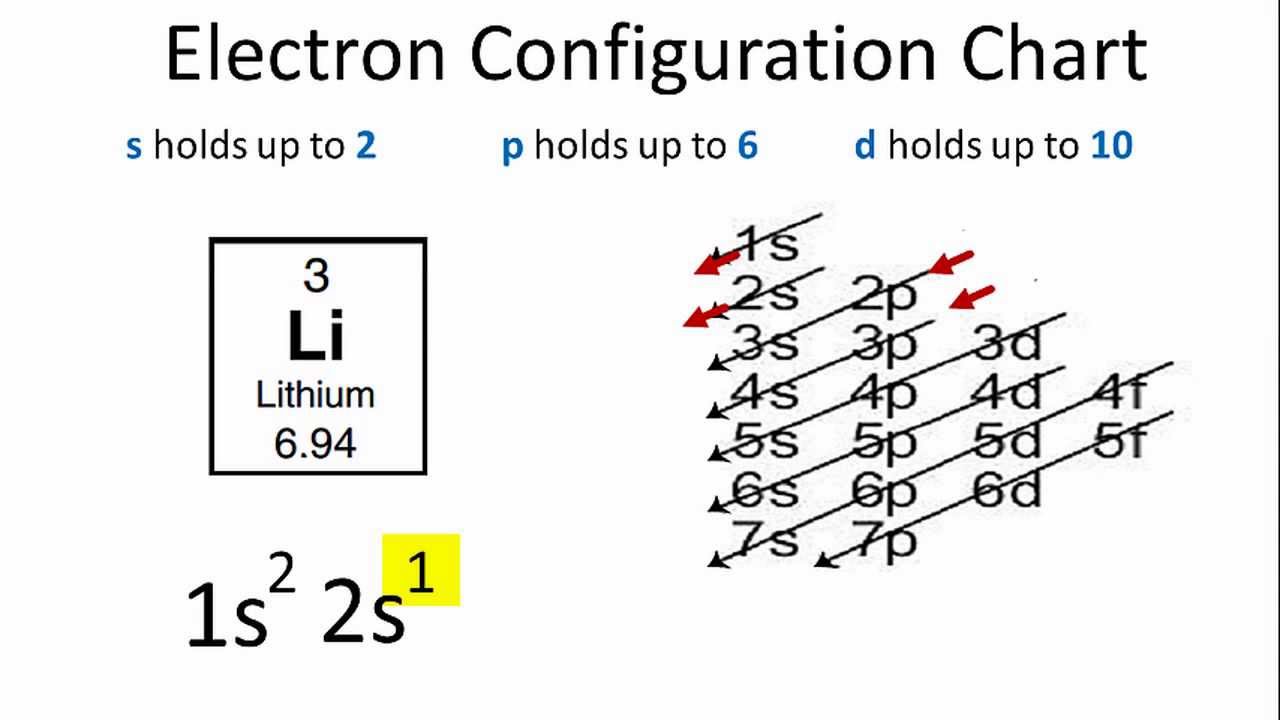
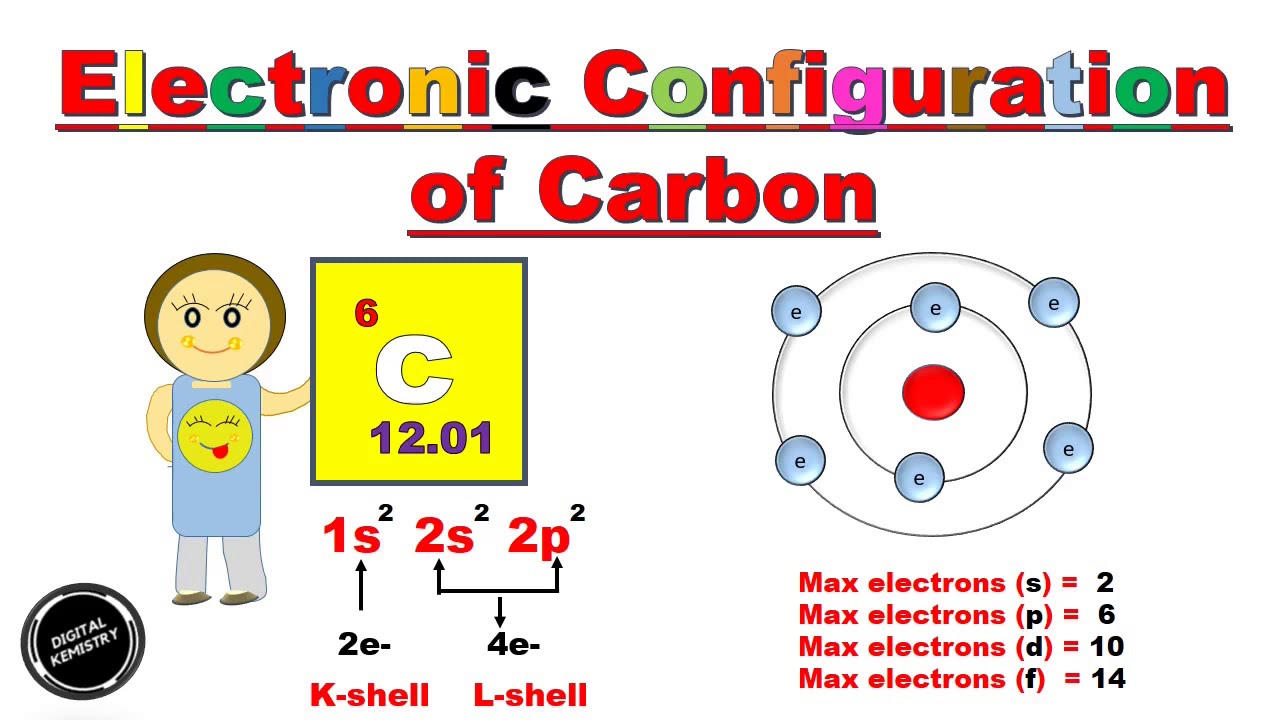
Carbon is the sixth element with a total of 6 electrons. In writing the electron configuration for carbon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for C goes in the 2s orbital. The remaining two electrons will go in the 2p orbital.
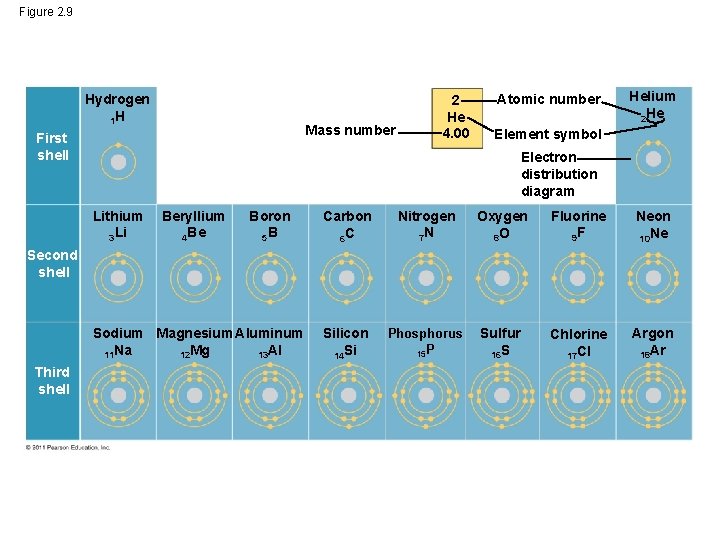
For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left.
Make an electron distribution diagram of carbon.! Carbon has 4 valence electrons, can bond to 4 items, and typically forms covalent bonds with other elements. 4. Carbon chains form skeletons. List here the types of skeletons that can be formed. Carbon skeletons vary in length. The skeleton may have double bonds, which can vary in location.
Make an electron distribution diagram of carbon. It is essential that you know the answers to these questions: How many valence electrons does carbon have? 4. How many bonds can carbon form? 4. What type of bonds does it form with other elements? Covalent bonds. Carbon chaines form skeletons. List the ypes of skeletons that can be formed.
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n
Electron Distribution Diagram Of Carbon Electron Configuration Chemistry Lessons Chemistry Help. SAVE IMAGE. The First Image Ever Of A Hydrogen Atom S Orbital Structure Physics World Hydrogen Atom Physics. SAVE IMAGE. Pin On Nucleophilic Substitution Reaction Sn1 And Sn2. SAVE IMAGE . Difference Between Polar And Nonpolar Molecules Definition Formation Properties Examples Covalent Bonding ...
The electron configuration of oxygen in Hund's principle is 1s 2 2s 2 2p x2 2p y1 2p z1. The electron configuration of oxygen in excited state is O* (8) = 1s 2 2s 2 2p x2 2p y1 2p z1. The last orbital of oxygen is 'p'. And unpaired electrons exist in its last p-orbital. So, the oxygen atom supports the Hund principle.
In normal condition the electronic configuration of carbon is : 1s2 2s2 2px1 2py1 2pz0. In excited state : 1s2 2s1 2px1 2py1 2pz1.7 answers · 15 votes: Electronic configuration of Carbon(C6) is [math]1s^{2},2s^{2},2p^{2}[/math] (ground state) ...
Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!)
Electron distribution diagram of carbon. Find out more in this video. Make an electron distribution diagram of carbon. Lewis who introduced it in his 1916 article the atom and the molecule. It is essential that you know the answers to these questions. Carbon is the sixth element with a total of 6 electrons. The distribution of electrons in the atoms electron shells know how to find the number ...
The second diagram corrects this by realizing there are two unused p orbitals on the carbon. The valence electron configuration of "O" is ["He"] 2s^2 2p^4. To accommodate the two lone pairs and the bonding pair, it will also form three equivalent sp^2 hybrid orbitals.
Question: 2. Draw an electron-distribution diagram of a carbon atom Show the correct numbers, and approximate locations, of all of this atom's subatomic particles. 2a. Use the following information for your drawing: Atomic number = 6 Mass number - 12 . This carbon atom is neutral 2b.
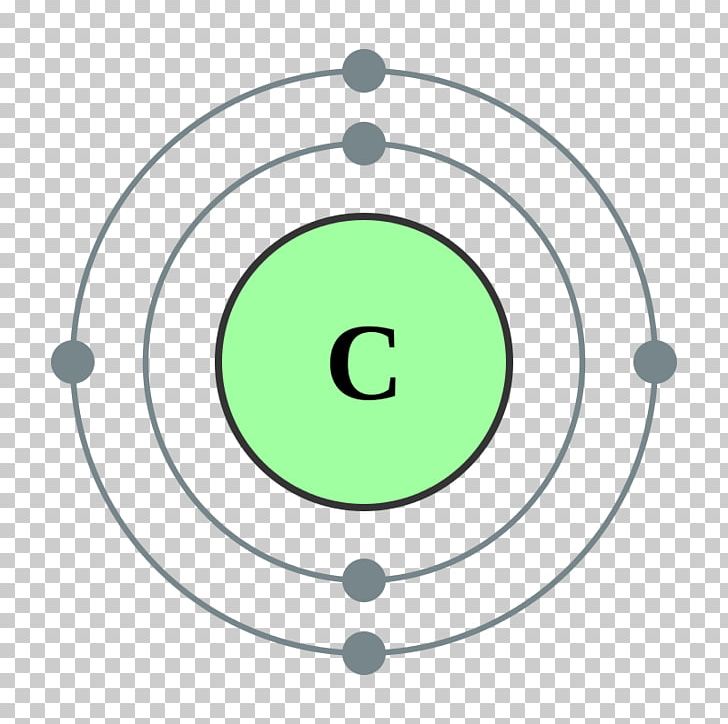
According to the Bohr diagram of Carbon, the outer shell is L-shell which contains 4 valence electrons. Properties of Carbon It belongs to Group 14 and period 2 in the periodic table. It is nonmetallic and tetravalent in nature. It has an electronegativity of 2.55 according to the Pauling scale. The common oxidation state of carbon is +4 and +2.
Each row on the periodic table introduces a new value for the principal quantum number n, while l goes as 0, 1, . . . , n-1. Recall that l = 0 -> s orbital, and l = 1 -> p orbital. Therefore, we would have these orbitals available: "Row 1:" 1s "Row 2:" 2s, 2p "Row 3:" . . . "Row 4:" . . . Carbon has access to only n = 2 and n = 1, so its six electrons can only go into the 1s, 2s and 2p ...
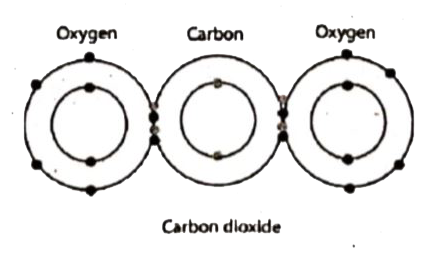
A Lewis electron dot diagram ... Note that carbon dioxide has two covalent bonds between each oxygen atom and the carbon atom, which is shown here as two lines and referred to as a double bond. When molecules are symmetrical, however, the atoms pull equally on the electrons and the charge distribution is uniform. 38 Related Question Answers ...
of carbon down to room temperature. The distribution of carbon was then studied over a cross section of the material using electron probe microanalysis. The migration of car-bon during the quenching process is estimated using numerical and analytical methods to evaluate how well the distribution of carbon was preserved through the quenching ...
Make an electron distribution diagram of carbon. Greg robsoncc by 20. Carbon skeletons vary in length. How many bonds can carbon form. The electron shells are shown moving outward from the nucleus. Sodium greg robsoncc by 20. C 12 c 13 and c 14 are isotopes of the element carbon. Note that the last term in the carbon electron configuration will be 1s2 2s2 2p2. Carbon chains form skeletons. In ...
Carbon is a group VIA element in the periodic table and contains six electrons in its last shell. Now, we know how many electrons are there in valence shells of carbon and oxygen atoms. valence electrons given by carbon atoms = 4 * 1 = 4 valence electrons given by oxygen atoms = 6 * 1 = 6 Total valence electrons = 10 Total valence electrons pairs
Such structure helps in understanding the arrangement of atoms along with the electrons participating in the bond formation. Now that you know how the Lewis structure is drawn and its uses let us quickly look at the CO2 Lewis structure. In CO2, the Carbon atom is in the central position as it is the least electronegative atom in the molecule.
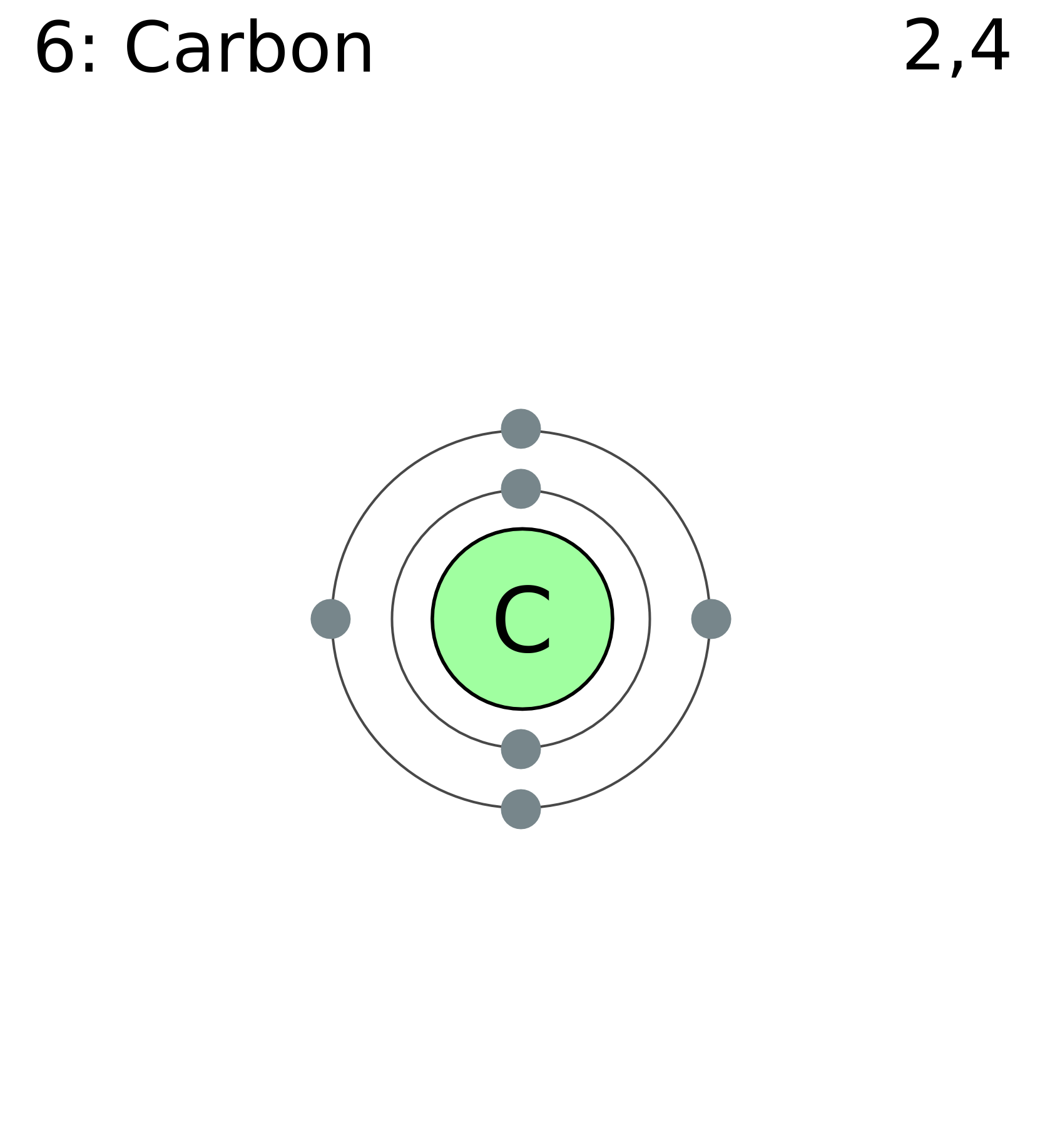
The electronic configuration of carbon is C (Z = 6) = 1s 2 2s 2 2p 2 The distribution of electrons in the carbon atom is as follows: In the first orbit or K-shell = 2 electrons In the second orbit or L-shell = 4 electrons Or, we can write the distribution of electrons in a carbon atom as 2, 4. Sodium
Transcribed image text: Some Scientists think that life elsewhere in the universe might be based on the element silicon, rather than on carbon, as on Earth. Look at the electron distribution diagram for silicon, and draw the Lewis dot structure for silicon on your note. Analyze your knowledge about it and propose a short essay about the possible silicon-based life.
Carbon dioxide \(\left(\ce{CO_2} \right)\) is a linear molecule. Oxygen atoms are more electronegenative than carbon atoms, so there are single bipedals that point out from the atom \(\ce{C}\) to each atom \ ... Electron distribution diagram of water which element is more electronegative ...
Electron distribution diagram of carbon. For each electron shell atom diagram the element symbol is listed in the nucleus. Carbon is the sixth element with a total of 6 electrons. The skeleton may have double bonds which can vary in location. Carbon skeletons vary in length. Carbon resulting in molecules that are mirror images of each other and thus. Make an electron distribution diagram of ...
Carbon 6C Silicon 14Si Nitrogen 7N Phosphorus 15P Oxygen 8O Sulfur 16S Fluorine 9F Chlorine 17Cl Neon 10Ne Argon 18Ar Helium 2He 2 He Mass number 4.00 Atomic number Element symbol Electron distribution diagram Fig. 2.9: Electrons are distributed in shells of orbitals. Each orbital contains a maximum of two electrons.
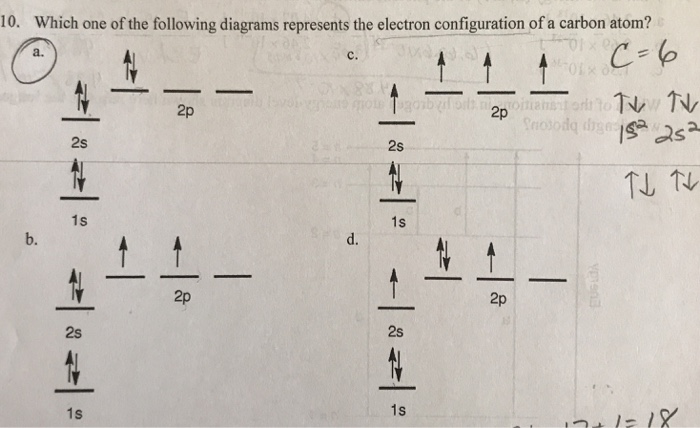
8 May 2021 — By Hund's rule, the electron configuration of carbon, which is 1s2 2s2 2p2, is understood to correspond to the orbital diagram shown in c.
The electron dot diagram of an element or a molecule is called Lewis structure; it features the distribution of valence electrons around elements. Carbon has four valence electrons and therefore, they are drawn on the four sides of a carbon atom as represented in the figures below. Answer link
Electron Distribution Diagram Of Carbon. Posted on December 10, 2018 December 10, 2018. Sponsored links. Related posts: Seymour Duncan Coil Tap Diagram. Mobile Network Booster Circuit Diagram. Vacuum Line Diagram. Hermetic Compressor Wiring Diagram. Moen Kitchen Faucet Parts Diagram. Posted in Diagram. Leave a Reply Cancel reply. Your email address will not be published. Required fields are ...
6 Sept 2021 — An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom.
Valence electrons of Carbon and Oxygen are represented as dots in the lewis diagram. To find the valence electron of carbon and oxygen we need the help of a periodic table. By looking at the periodic table, we come to know carbon belongs to 14 groups and oxygen belongs to the 16th group in the periodic table. Hence, carbon has 4 valence ...
14 Nov 2019 — Carbon requires four more electrons and oxygen requires two more electrons to get the octet state. So that each oxygen shares two electrons with ...2 answers · 9 votes: your answer is in attachment
Electron Configuration Chart for All Elements in the Periodic Table. There are 118 elements in the periodic table. Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom.
... Abundance, Physical Properties, Thermal Properties, Crystal Structure, Atomic & Orbital Properties, electron configuration, Chemical Properties carbon, ...
In the same vein, the partial charge distribution of Li adatoms and carbon atoms of the edge of the MV defect also supported electron transfer between them (Supplementary Fig. 13). Therefore, the ...



















:max_bytes(150000):strip_icc()/carbonatom-58b602855f9b5860464c8bf6.jpg)





0 Response to "44 carbon electron distribution diagram"
Post a Comment