41 lewis diagram for n2
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation December 31, 2015 - Answer (1 of 3): As nitrogen is in fifth group in periodic table therefore it will have five electrons in the valance shell in which three electrons are unpaired because it needs three electrons to complete its outermost shell. Therefore in case of N2, each nitrogen atom will share three electron...
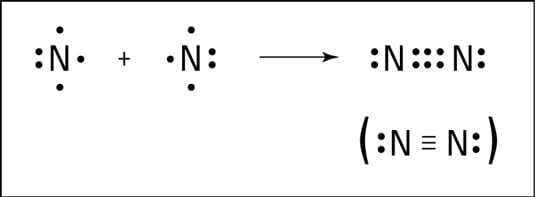
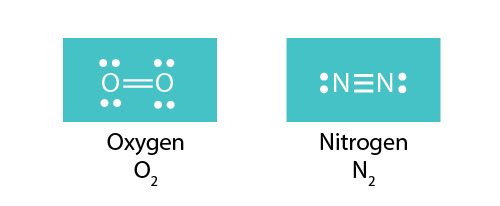
In the lewis structure of N2, there is a triple bond between two nitrogen atoms. The molecular geometry of N 2 is linear. N2 is colorless, odorless, and tasteless gas. Each nitrogen atom is surrounded by a lone pair of electrons. There are three half-filled 2p orbitals in the valence shell of the nitrogen atom.
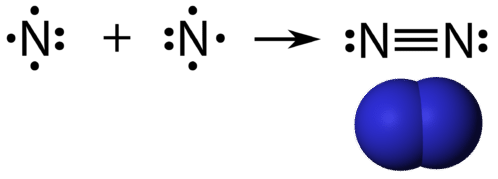
Lewis diagram for n2
What is the Lewis dot diagram for nitrogen? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable. Lewis Structure of N2 (Nitrogen gas)||Lewis Structure for N2 (Nitrogen gas)#LewisStructureofN2#N2LewisStructure#LewisStructureforN2This video has solved the ... How to Draw the Lewis Structure of N2 - with explanation!Check me out: http://www.chemistnate.com
Lewis diagram for n2. Nov 19, 2018 · on Lewis Dot Diagram For N2. Plucked this image from google. There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple. The Lewis Structure for N2 looks easy at first. The problem is that there aren't enough valence electons to give both Nitrogen atoms an octet. You'll need to use . Considering the energy level diagram, the configuration of N2 is σ1S2, σ *1S2, σ2S2, σ*2S2, π2Px2, π2Py2, σ2Pz1. Conclusion In the Lewis structure of the N2 molecule, there is a development of a triple covalent bond stood for by three lines in between two atoms of Nitrogen. Answer (1 of 2): In exactly the same way as you'd do it for any simple, covalently bonded compound. First you need to know the valency of each element. Then you need to draw a stick model with bonds between the atoms, so that each atom's valency is satisfied (so N has three bonds coming out of i... Solution for Draw the Lewis Dot structure for N2 (on paper, not on Canvas) then answer the questions. a. How many total valence electrons are in N2? Express…
A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t... November 14, 2020 - It’s easiest to think in terms of dots to make the N2 Lewis structure. · Nitrogen needs to bond three times, shown as the lone dots on the left, right and bottom of the N atoms in the below diagram. There is also a pair of dots, representing two more electrons, that won’t bond, on top of each N. Think of connecting the lone dots to form ... Magnesium nitride (Mg3N2 or N2Mg3) is IONIC because it is a combination of a metal and non-metal.Each magnesium, of which there are three, LOSES two electron... Drawing the Lewis Structure for N 2 (Dinitogen or Nitrogen Gas). Nitrogen (N 2) is a commonly tested Lewis structure due to its importance on Earth (about 78% of the Earth's atomsphere is N 2).It also is a good example of a molecule with a triple bond. There are 10 valence electrons available for the Lewis structure for N 2.. Video: Drawing the Lewis Structure for N 2
N2 Lewis structure ... In the Lewis structure of There are three bonds shown as the three parallel lines between the N atoms. This is a triple bond, with each ... The N 2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair. Lewis Structure for NO 2 | Nitrogen dioxide Oxidation number. Lewis structure of NO 2 (Nitrogen dioxide) is drawn in this tutorial step by step. You can learn basics of how to draw a lewis structure properly from this example. This is a special case of lewis structure drawing because, there is a unpaired electron on nitrogen atom. In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
Only the outer electrons are shown in the dot and cross Lewis diagram of nitrogen below. (c) doc b. :N≡N: is the displayed formula for nitrogen showing the ...
A step-by-step explanation of how to draw the N2O Lewis Dot Structure (Dinitrogen monoxide or Nitrous Oxide).For the N2O structure use the periodic table to ...

Next stop...our favourite padstal - Thyme and Again. We stop here every time we drive through Plett, their coffee is great and they make the best Carrot Cake I've tasted (next to my sisters - hers is the absolute best.)
A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Diatomic Nitrogen).For the N2 structure use the periodic table to find the total number...
Answer (1 of 3): As nitrogen is in fifth group in periodic table therefore it will have five electrons in the valance shell in which three electrons are unpaired because it needs three electrons to complete its outermost shell. Therefore in case of N2, each nitrogen atom will share three electron...
May 10, 2017 - Something went wrong. Wait a moment and try again
The prerequisite courses are General ... energy), Lewis symbols and structures, ionic bonding and energetics, covalent bonding, molecular geometry, VSEPR, bonding theories (valence bond theory, hybridization), covalent bonding and orbital overlap, molecular orbitals for diatomic molecules, gas laws, phase changes, phase diagrams, structures ...
Answer: The Lewis structure for Nitrogen is as follows You can see the triple bond with the lone pair on both of the nitrogen atoms. The molecular orbital diagram for nitrogen is as follows You can see the accounting for each of the valence electrons (5 from each atom) place them into three bo...
Lewis structure of N2 A Lewis Structure is a very simple representation of the valence, or outermost, electrons in a molecule. It does not explain the geometry of the molecule, but it is a step forward in approaching the geography. But to find out if N2 is polar or nonpolar, the Lewis Structure can reveal the best electron makeup of the molecule.
The N2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair.
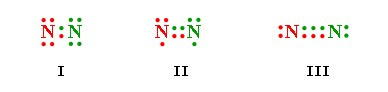
Click here to get an answer to your question ✍️ Which of the following represent the lewis structure of N2 molecule?
for 3 dage siden ... N2 Lewis Structure Hybridization and Atomic Math. Nitrogen (N2) is a dull, scentless, bland gas and is the most plentiful component in Earth's ...
Answer to draw the Lewis structure for N2. be sure to draw all bonds and lone pairs ...
for 6 dage siden ... N2 Lewis dot structure have three bonds like three parallel lines between the N atoms. Each bond in this structure is produced by a pair of ...
Nitrogen (N 2) Molecule Lewis Structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure.. N 2 lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.
cactus
Lewis Dot of Dinitrogen (Nitrogen Gas) · Back70 More Lewis Dot Structuresfrom-http://en.wikipedia.org/wiki/Dinitrogen-Nitrogen is a nonmetal, with an electronegativity of 3.04. It has five electrons in its outer shell and is therefore trivalent in most compounds.
In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. Here is the electron dot structure for a single N atom: The total number of valence electrons between the two N atoms is 10 e^-.
Transcript: For the N2 Lewis structure, we have five valence electrons for Nitrogen--it's in group 5 or 15 on the periodic table. We have two Nitrogens. Multiply those together, we have a total of 10 valence electrons for the N2 Lewis structure. We'll put the two Nitrogens next to each other, and then we'll put two valence electrons between them to form a chemical bond.
Sep 23, 2020 - A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic ...
Lewis dot diagram for n2. 25 it has five electrons in its outermost valence shell. If you are talking about the lewis dot diagram then n 2 would have 5 dots around each of the letter ns so that there would be 6 dots total what is the lewis dot structure for n2 look like.
March 1, 2018 - Answer: N2 is non polar with 10 valence electrons. Triple bond between the N atoms and a non bonded electron pair on each N. Each N is sp hybridized.
The Lewis structure of the following compound is given by: The molecular geometry of nitrogen molecule is linear. This compound is non-polar in...
I quickly take you through how to draw the Lewis Structure of N2 (DiNitrogen) . I also go over the shape and bond angles.

U.S. Representative John Lewis poses for a photo at Black Lives Matter plaza in Washington DC (IG: @clay.banks)
January 3, 2021 - What is the formula of nitrogen gas? How is n2 formed? The Lewis Structure or the Lewis Dot Structure or the Lewis Dot Diagram, named after Gilbert N. Lewis, shows the diagram of the atomic bonding of the molecules or an element. It shows the lone pairs of molecules existing in a molecule.
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
Based on the Lewis electron-dot diagrams of N2 and N2H4, N2 has a stronger nitrogen-to-nitrogen bond than N2H4. The strength of a bond is dependent on the bond length and the bond order. The higher the bond order, the shorter and stronger the bond. Hence triple bonds are stronger than double bonds and double bonds are stronger than single bonds.
Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell. As per the molecule N2, it has two atoms of Nitrogen.
KCET 2006: Which of the following represents the Lewis structure of N2 molecule ? (A) Image A (B) Image B (C) Image C (D) Image D. Check Answer and So.
Lewis dot structure of n2 molecule Let's take a look at the Lewis of and N2 structure. Atomic nitrogen has 5 valence electrons and 4 orbital valence (2s, 2 px, 2py and 2pcs). In the Lewis structure there is a triple link between nitrogen atoms and a pair of non-binding electrons on each. This is consistent with the physical properties of N2.
The N 2 chart, also referred to as N 2 diagram, N-squared diagram or N-squared chart, is a diagram in the shape of a matrix, representing functional or physical interfaces between system elements. It is used to systematically identify, define, tabulate, design, and analyze functional and physical interfaces. It applies to system interfaces and hardware and/or software interfaces.
How to Draw the Lewis Structure of N2 - with explanation!Check me out: http://www.chemistnate.com
Lewis Structure of N2 (Nitrogen gas)||Lewis Structure for N2 (Nitrogen gas)#LewisStructureofN2#N2LewisStructure#LewisStructureforN2This video has solved the ...
What is the Lewis dot diagram for nitrogen? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable.







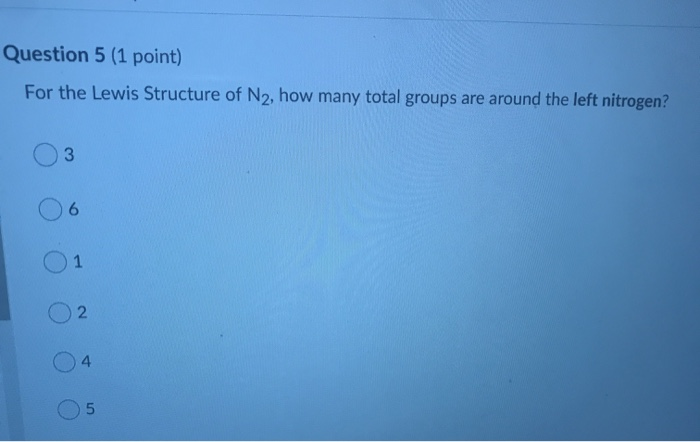

















0 Response to "41 lewis diagram for n2"
Post a Comment