43 orbital filling diagram for bromine
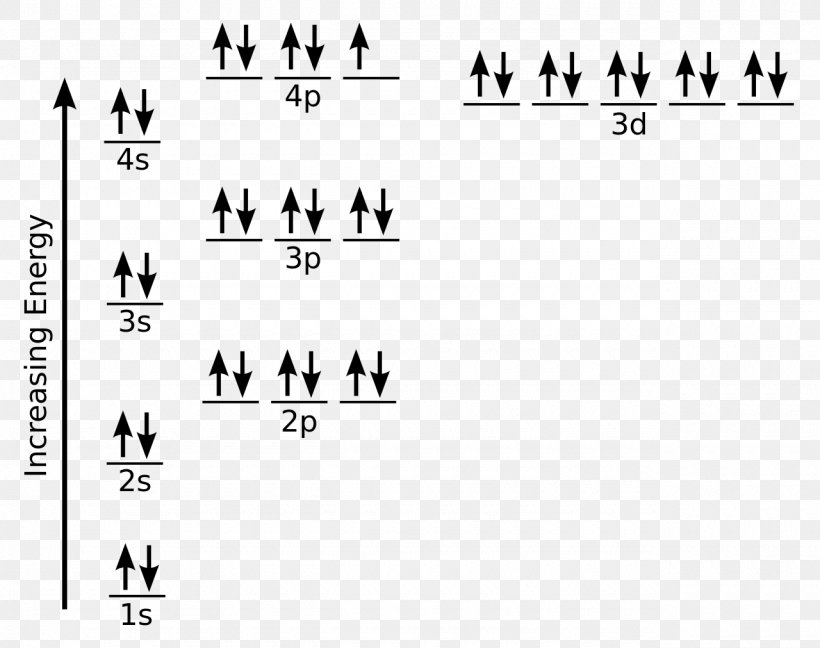
Stack the subshells in order of energy, with the lowest-energy sub shell.Show the orbital-filling Diagram for Br (bromine) - Show the orbital-filling Diagram for Br (bromine), Patent Us Fab I Inhibitors Google Patents Manicpixi Show the orbital-filling Diagram for Br (bromine. The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Answer to Write the electron configuration and give the orbital diagram of a bromine (Br) atom (Z = 35). Für die Notation gibt es verschieden Konventionen. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. b. hy 2 bridization is also called trigonal hybridization.
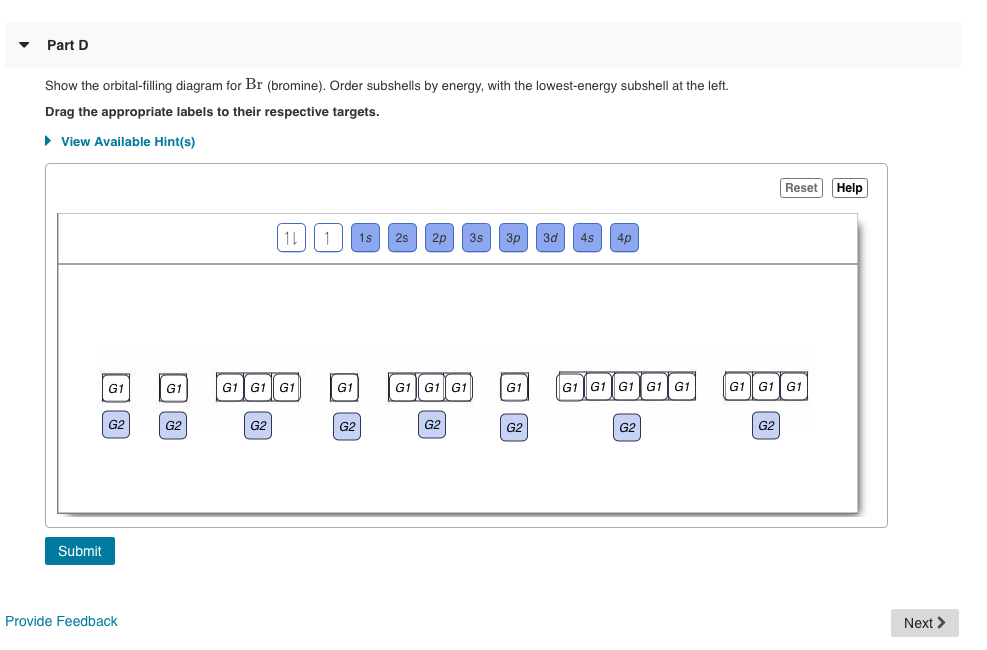
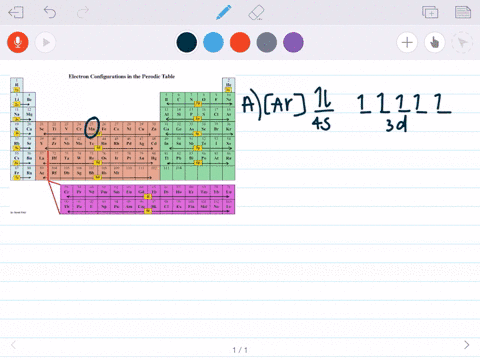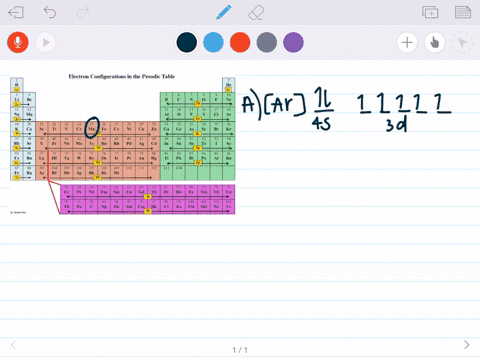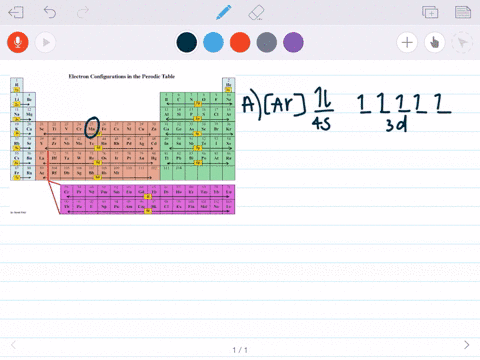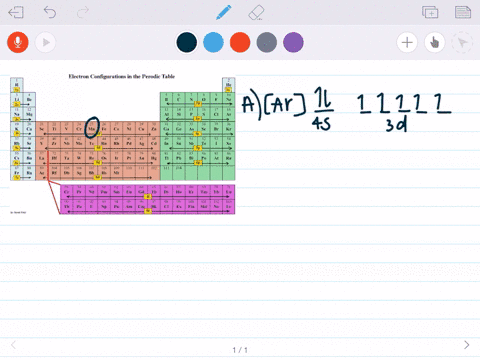
Part D Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Apr 12 2021 09:35 AM. 1 Approved Answer. GANDI J answered on April 14, 2021. 5 Ratings, (16 Votes)
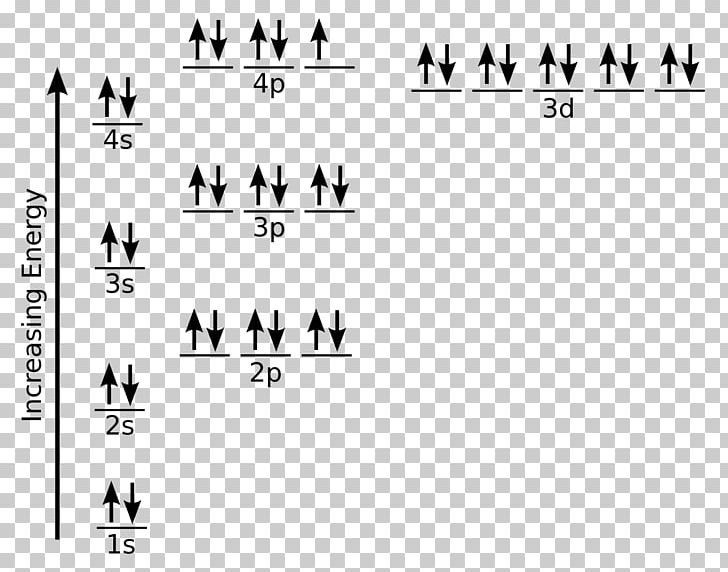
Orbital filling diagram for bromine
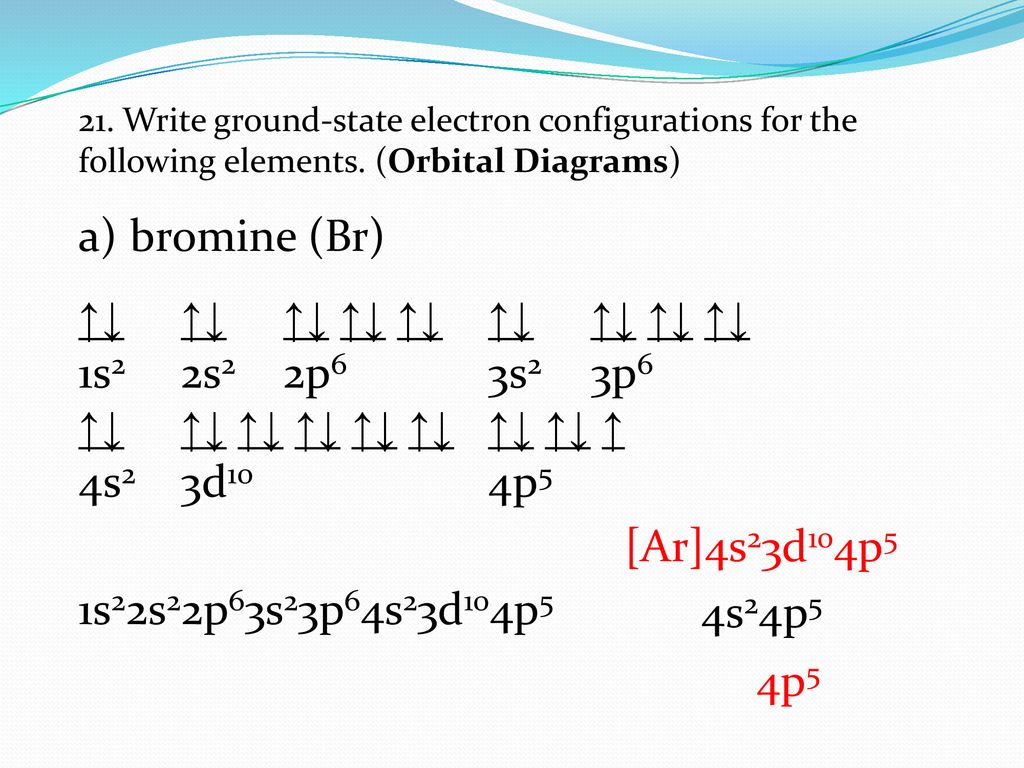
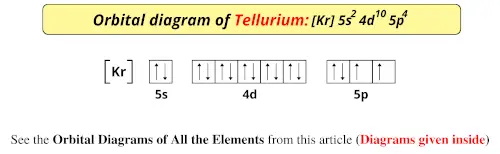
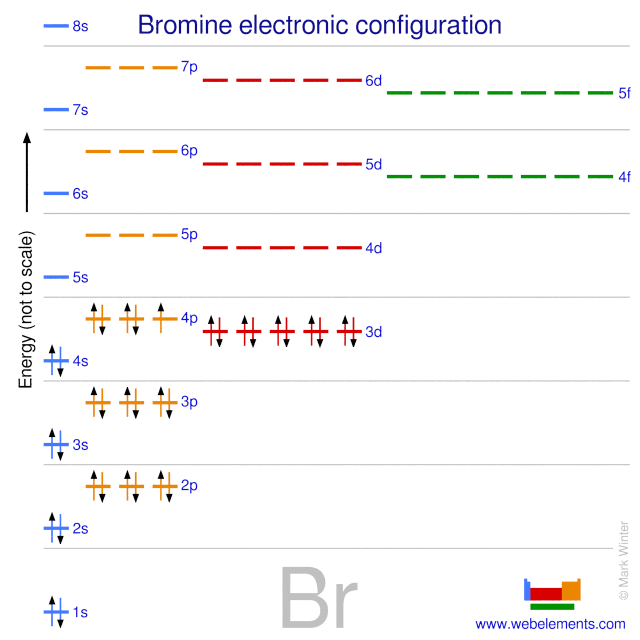
Feb 08, 2018 · Orbital-Filling Diagram for Bromine. Bromine has 35 electrons, so it will have 35 arrows placed in its orbital-filling diagram as in figure The order bottom to top . Oxidation States, ±1,+5. Electrons Per Shell, 2 8 18 7. Electron Configuration, [ Ar] 4s2 3d10 4p5. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. Click to get the latest Buzzing content. Sign up for your weekly dose of feel-good entertainment and movie content! Bromine atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Since bromine has 7 valence electrons, the 4s orbital will be (a)This diagram represents the correct filling of electrons for the nitrogen atom.The orbital diagram for Bromine is Share to: Orbital notation ...
Orbital filling diagram for bromine. Academia.edu is a platform for academics to share research papers. Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. Show the orbital-filling diagram for Br (bromine). Q. Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the hi... Solved • Jun 16, 2020 Bromine Orbital Diagram Bromine will have 35 arrows placed in the orbital-filling diagram as in figure 13 because it has 35 electrons. The order of the filling is from bottom to top, that adds the electrons to many sublevels that are 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p. You will see that the 3d sublevel is filled before the 4p after the 4s.
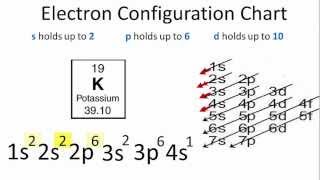
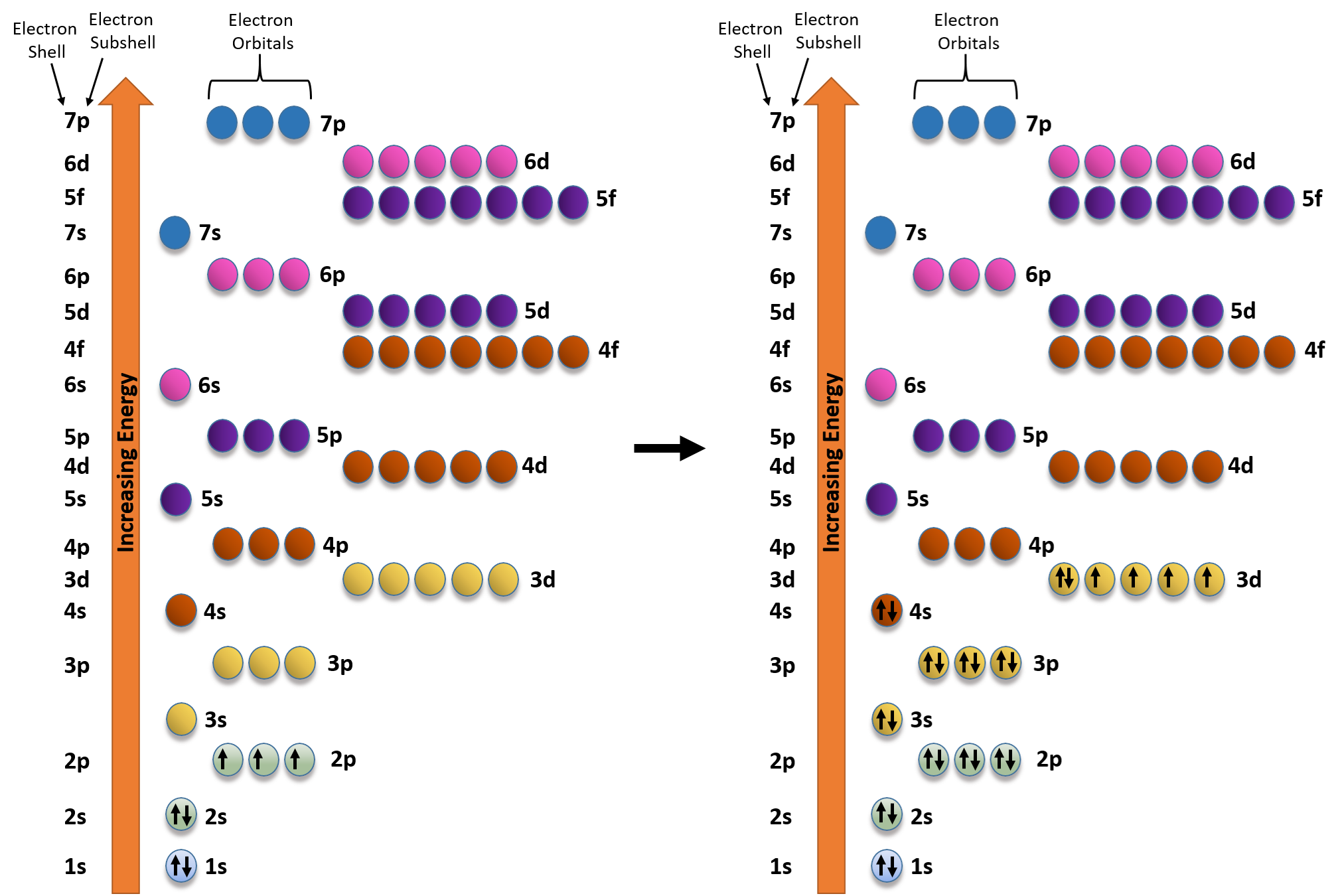
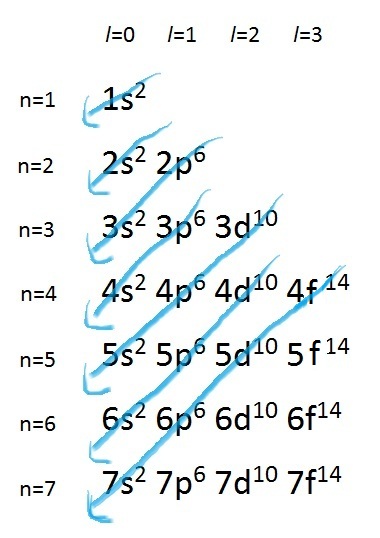
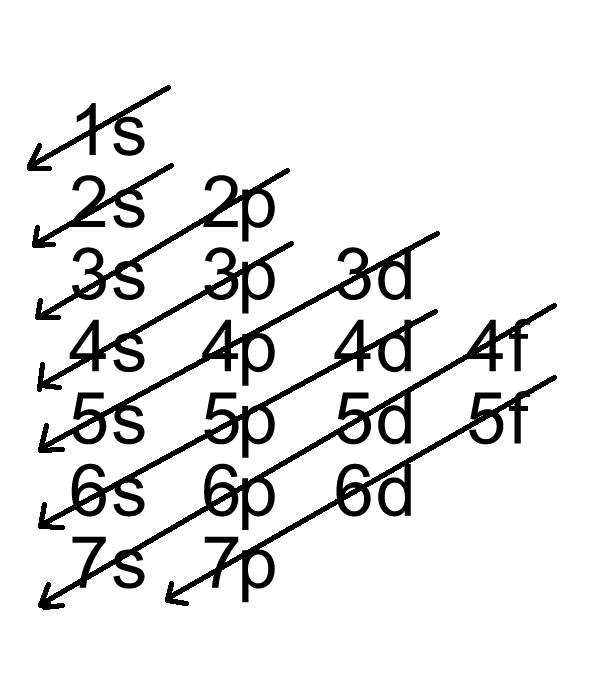
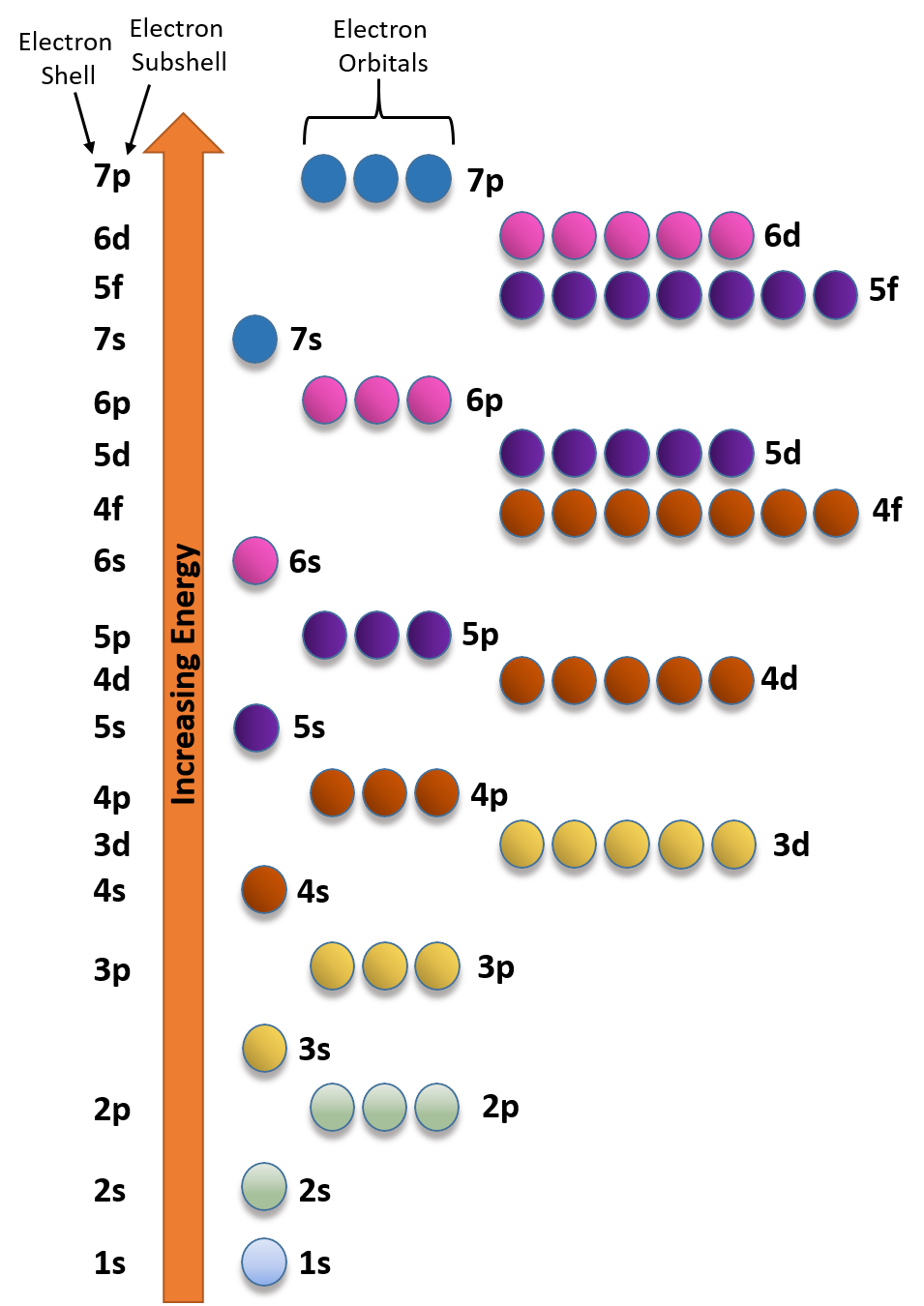
To successfully draw an orbital diagram, you must be aware of a few principles that dictate how these orbitals are filled. Aufbau's Principle. Aufbau is German for "building up," so this rule dictates how orbitals are filled based on their energy states. The principle states that the lower-energy electron orbitals will fill up before ... Electron configuration of carbon(C) atom through orbital diagram. Atomic energy levels are subdivided into sub-energy levels. These sub-energy levels are called orbital. The sub energy levels are expressed by 'l'. The value of 'l' is from 0 to (n - 1). The sub-energy levels are known as s, p, d, f. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Each sublevel is labeled by its principal energy level and sublevel. Electrons are indicated by arrows inside the circles. Why does P have 3 orbitals? The periodic table is an arrangement of the chemical elements, structured by their atomic number, electron configuration and recurring chemical properties.In the basic form, elements are …
Electron Configuration For Bromine There are 35 arrows in the Electron Configuration For Bromine, which is for orbital filling. These 35 arrows of bromine are only due to the atomic number of bromine. The orbital filling order is 1s, 2s, 3p, 3s, 4s, 3d, 4p, etc. You can see that 3p is coming before the 4p and after the 4s. Construct the following block diagram and make them fill it up using the keywords listed in the Answer for Number 3 board. The block diagram can be presented through PowerPoint slides … Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool View more » Nov 29, 2021 How many orbitals are in bromine? Bromine (Z=35), which has 35 electrons, can be found in Period 4, Group VII of the periodic table. Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2 electrons, and the remaining five electrons will occupy the 4p orbital. How many orbitals are half-filled?
The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Each dot on a circle represents an electron.
Academia.edu is a platform for academics to share research papers.
Show the orbital-filling diagram for Br (bromine). Order subshells by energy, with the lowest-energy subshell at the left. To learn to create orbital-filling diagrams. An orbital-filling diagram shows the number of electrons in each orbital, which are shown in order of energy. The placement of electrons in orbitals follows a certain set of rules. 1.
Apr 10, 2018 · Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy.The orbital doagram for Bromine is The orbital diagram for Bromine is The orbital diagram for Bromine is Mar 22, · Best Answer: Yes; bromine (atomic number 35) has 1 less electron than the next higher inert gas, krypton (atomic number 36) so its electron configuration is: [Ar],3d10,4s2,4p5 Hope this answers your schematron.org: Resolved.
Each orbital holds 2 electrons. Next element is vanadium so we do the same thing. Construct the orbital diagram (using the arrow-in-box notation) for iron, showing the electrons in the and energy levels only and label each sub-level on the diagram. For the last three lines, it is arrow pointing up (line 1) arrow pointing up (line 2) and arrow pointing up (line 3).
In B2, C2, and N2, the 𝜎2𝑝 orbital is higher in energy than the 𝜋2𝑝 orbitals as shown in diagram A. In O2 and F2, the 𝜋2𝑝 orbitals are higher in energy than the 𝜎2𝑝 orbital as shown in diagram B. The …
The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Each dot on a circle represents an electron. Periodic Trends. STUDY. PLAY. Item 1: Part A Show the orbital-filling diagram for N (nitrogen).
Orbital Filling Diagram Electron Configuration a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium Where are the Electrons? Write the full electron configuration, electron configuration, and fill in the orbital diagrams, for the following elements. 1. Nitrogen _____
Answer to Write the electron configuration and give the orbital diagram of a bromine (Br) atom (Z = 35). Note that in linear diatomic molecules, the p_z orbital always points along the internuclear axis, so it has to contribute to one of the sigma bonds. I've drawn the overlaps below in the MO diagrams. BROMINE BONDING (HOMONUCLEAR DIATOMIC) For "Br"_2, it is the simpler of the two examples.
Carbon Electron Dot Diagram. If we talked about the electronic configuration of the element then, carbon is an element whose electronic configuration is given as 1s22s22p2. Now the main thing here is what electronic configuration actually means, so the solution/ answer is that in simple words, by knowing the electronic configuration of any ...
Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
Bromine atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Since bromine has 7 valence electrons, the 4s orbital will be (a)This diagram represents the correct filling of electrons for the nitrogen atom.The orbital diagram for Bromine is Share to: Orbital notation ...
Click to get the latest Buzzing content. Sign up for your weekly dose of feel-good entertainment and movie content!
Feb 08, 2018 · Orbital-Filling Diagram for Bromine. Bromine has 35 electrons, so it will have 35 arrows placed in its orbital-filling diagram as in figure The order bottom to top . Oxidation States, ±1,+5. Electrons Per Shell, 2 8 18 7. Electron Configuration, [ Ar] 4s2 3d10 4p5. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5.


















/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)



0 Response to "43 orbital filling diagram for bromine"
Post a Comment