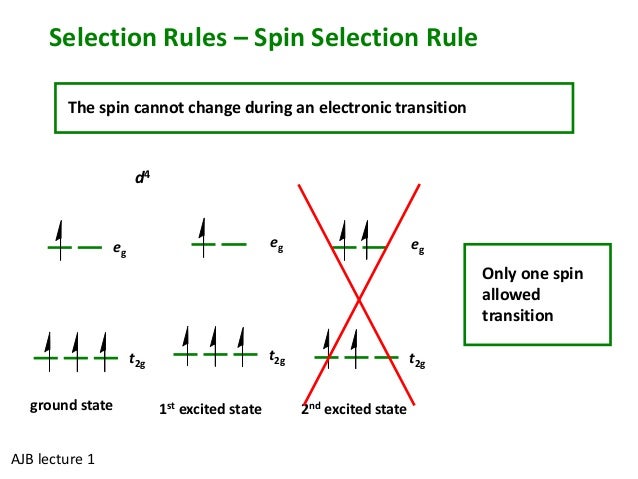
44 v5+ orbital diagram
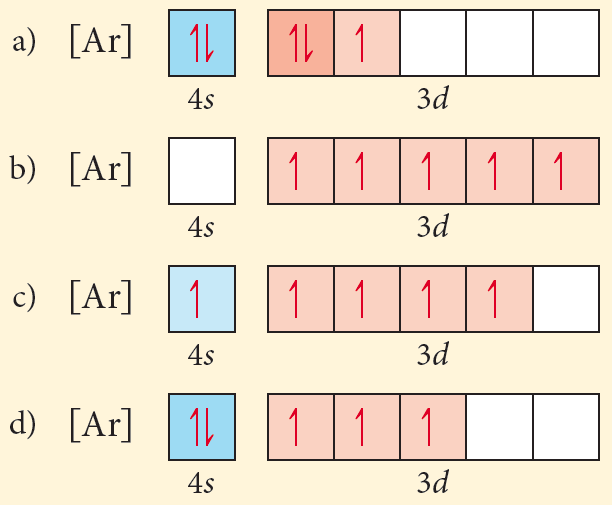
V5+ orbital diagram. V5+ Orbital Diagram. Since the 4s orbital is higher in energy, its electrons will be removed first. Not that it matters here, though, because exactly 5 electrons are. can be accommodated in the metal d orbitals. • d0 ions – Ti4+, Zr4+, V5+, Ta5+, Cr6+, Mo6+, etc. • d1 ions . σ-ML4 Tetrahedral MO Diagram e. Orbital Diagram. Orbital diagrams are ways to assign electrons in an atom or ion. Each atomic orbital is represented by a line or a box and electrons in the ...1 answer · Top answer: 1. The orbital diagrams are shown in the figures below. 2. V5+V5+ has all the electrons paired up so it is...
Chemistry Q&A Library Write orbital diagram for V5+ Write orbital diagram for V5+ close. Start your trial now! First week only $4.99! arrow_forward. Question. Write orbital diagram for V 5+ check_circle Expert Answer. Want to see the step-by-step answer? See Answer. Check out a sample Q&A here.
V5+ orbital diagram
V5+ Orbital Diagram. Since the 4s orbital is higher in energy, its electrons will be removed first. Not that it matters here, though, because exactly 5 electrons are. can be accommodated in the metal d orbitals. • d0 ions – Ti4+, Zr4+, V5+, Ta5+, Cr6+, Mo6+, etc. • d1 ions . σ-ML4 Tetrahedral MO Diagram e. 9. Electrons in an orbital with l = 3 are in a/an. A) d orbital. B) f orbital. C) g orbital. D) p orbital. E) s orbital. 10. The number of orbitals in a d subshell is. A) 1 B) 2 C) 3 D) 5 E) 7. 11. "No two electrons in an atom can have the same four quantum numbers" is a statement of. A) the Pauli exclusion principle. D) de Broglie's relation. Write orbital diagrams for each of these ions. V5+ Cr3+ Ni2+ Fe3+ Determine if the ion is diamagnetic or paramagnetic. [Ne] 3s^2 3p^6 [Ar] 4s^0 3d^3 [Ar] 4s^0 3d^8 [Ar] 4s^0 3d^5 diamagnetic: V5+ paramagnetic: Cr3+, Ni2+, Fe+. Choose the larger atom from each of the following pairs. Al or In
V5+ orbital diagram. Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V5+,Cr3+,Ni2+,Fe3+ Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Electron configurations help you to do this. To calculate an electron configuration, divide the periodic table into sections to represent the atomic orbitals, the regions where electrons are contained. Groups one and two are the s-block, three through 12 represent the d-block, 13 to 18 are the p-block and the two rows at the bottom are the f-block. Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V 86%(14). Write orbital diagrams for each of these ions. A. V^5+ B. Cr^3+ C. Ni^2+ D. Fe^3+ E. Determine if the following ions are diamagnetic or paramagnetic. V5+ orbital diagram keyword after analyzing the system ... The 1s orbital at the bottom of the diagram is orbital with electrons of the lowest energy. Zinc (Zn). So the only possible stable configuration would be 4s2 3d3. Fill in the orbital energy diagram for zinc ion The lowest E levels are already filled in for you . Write an orbital diagram for the state of the earth of the zinc atom.
Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. V5+ b. Cr3+ c. Ni2+ d. Fe3+ The VEX Robotics Design System offers students an exciting platform for learning about areas rich with career opportunities spanning science, technology, engineering and math (STEM). These are just a few of the many fields students can explore by creating with VEX Robotics technology. Beyond science and engineering principles, a VEX Robotics project encourages teamwork, leadership and problem ... Just like there are 5 valence electrons for the element Vanadium. Similarly, every element will have its own valence electrons and many more. You can refer our article to those users or your friends who are looking for the information related to the Vanadium Electron Configuration of valence electrons as the good thing about our article is that it is available free of cost and no charges are ... you. We have orbital diagrams for some kind of funky looking omens that have he's, uh, extreme lack of electrons minus five minus two minus three. And it is affecting if they are considered dia, magnetic or pair of magnetic if they are equally balanced. If there's a charge that's either morning making them tracked it to others or repelling themselves.
View GeneralChemistry1_Q2_Module-1_Quantum_Mechanical_Descriptions_v5-1.pdf from CHEM 111 111 at University of Cebu - Main Campus. Senior High School NOT General Chemistry 1 Quarter 2 - Module Transcribed image text: Part A Enter an orbital diagram for V5+ Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all targets will be filled Reset Help P 111 1s 25 38 4s 2p 3p 4p 3d 61 G1 G1 GIG1 G1 GIG1 G1 G1 G1G1G1 G1|| G1 G16161 62 G2 G2 G2 G2 G2 G2 G2 Submit Request Answer Part B Enter an orbital diagram for Cr3 Drag the ... View GeneralChemistry1_Q2_Module-2_Electron-Configuration-and-Magnetic-Property_v5-1.pdf from CCS 456 at University of Cebu - Main Campus. Senior High School NOT General Chemistry 1 Quarter 2 - What element is represented by this orbital diagram? Pauli Exclusion Principle. 2 electrons in the same orbital must have opposite spins. Hund's Rule. Electrons don't pair up in orbitals of equal energy until they have to, and all electrons in singly occupied orbitals have the same spin.
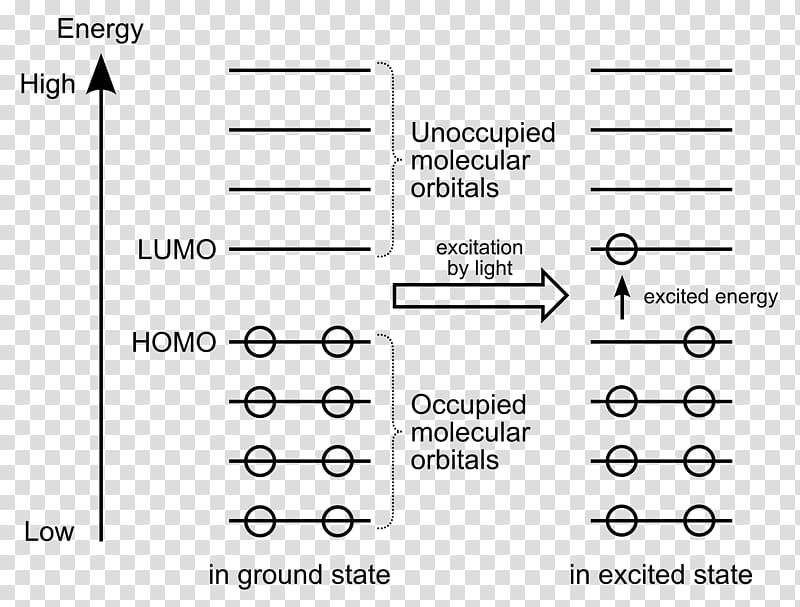
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of .. Rb+, Se2−. The first orbital (an s orbital) can contain only two electrons.. Rubidium.
Example of following the Aufbau principle, Pauli principle, and Hund's rule to construct an orbital diagram for a vanadium (Z=23) atom.
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Found in the minerals patronite (VS4), vanadinite [Pb 5 (VO 4) 3 Cl], and carnotite [K 2 (UO 2) 2 (VO 4) 2 .3H 2 O]. Vanadium is usually produced as a by-product of refining other ores and from Venezuelan oils. Annual world wide production is around 7,000 tons. Uses of Vanadium:
V5+ orbital diagram keyword after analyzing the system. 1. Write orbital diagrams for each of these ions. *a. V5. Study.com DA: 9 PA: 50 MOZ Rank: 60. Orbital diagrams are ways to assign electrons in an atom or ion. Each atomic orbital is represented by a line or a box and electrons ….
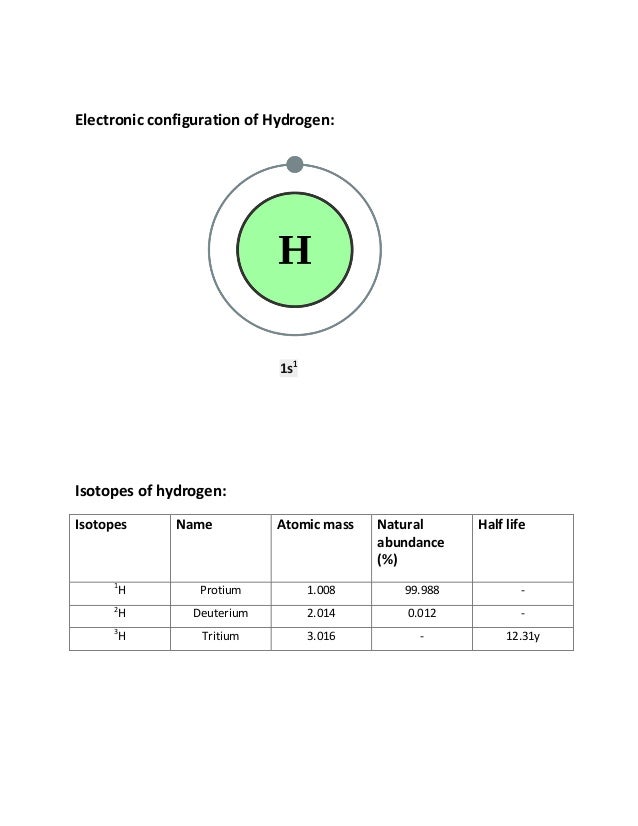
To write the configuration for the Vanadium and the Vanadium ion, first we need to write the electron configuration for just Vanadium (V). We first need to ...
Finance investment stock market chart. Made with analog vintage lens, Leica APO Macro Elmarit-R 2.8 100mm (Year: 1993)
Q. Write orbital diagrams for each of these ions.V5+ Q. Write orbital diagram to represent the electron configurations-without hybridization-for F in SF2. Q. Choose the correct orbital diagram for vanadium. See all problems in The Electron Configuration: Ions Frequently Asked Questions What scientific concept do you need to know in order to ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. It is sandwiched between the well-known elements zinc and germanium.
Now I asked to look, were asked also to look at making an orbit diagram and determining whether vanadium as a five plus cat iron is dia or para magnetic in ...4 answers · Top answer: take a look here at utilizing the periodic table too. Do some orbital configurations or diagrams ...
Write orbital diagrams for each of these ions. V5+ Cr3+ Ni2+ Fe3+ Determine if the ion is diamagnetic or paramagnetic. [Ne] 3s^2 3p^6 [Ar] 4s^0 3d^3 [Ar] 4s^0 3d^8 [Ar] 4s^0 3d^5 diamagnetic: V5+ paramagnetic: Cr3+, Ni2+, Fe+. Choose the larger atom from each of the following pairs. Al or In
9. Electrons in an orbital with l = 3 are in a/an. A) d orbital. B) f orbital. C) g orbital. D) p orbital. E) s orbital. 10. The number of orbitals in a d subshell is. A) 1 B) 2 C) 3 D) 5 E) 7. 11. "No two electrons in an atom can have the same four quantum numbers" is a statement of. A) the Pauli exclusion principle. D) de Broglie's relation.
V5+ Orbital Diagram. Since the 4s orbital is higher in energy, its electrons will be removed first. Not that it matters here, though, because exactly 5 electrons are. can be accommodated in the metal d orbitals. • d0 ions – Ti4+, Zr4+, V5+, Ta5+, Cr6+, Mo6+, etc. • d1 ions . σ-ML4 Tetrahedral MO Diagram e.

.png)






















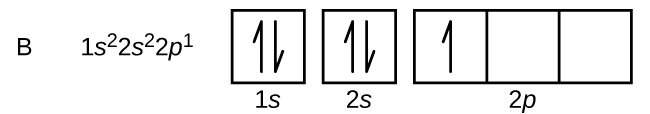
0 Response to "44 v5+ orbital diagram"
Post a Comment