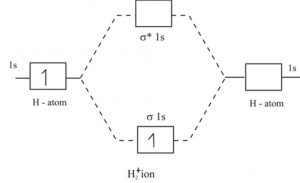
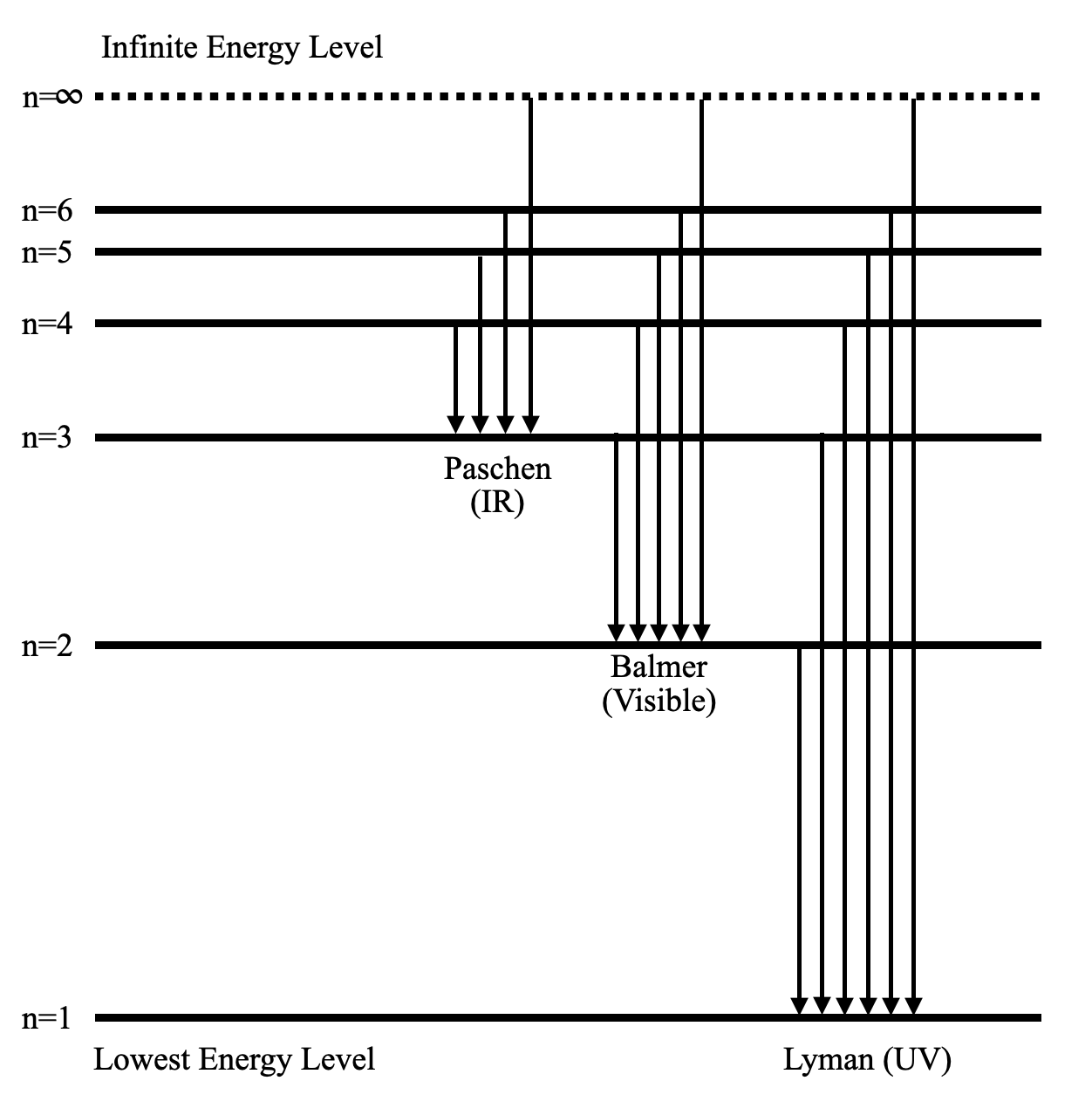
42 complete the energy-level diagram for h2−.
PDF Q1. The following table shows some enthalpy change and ... The free-energy change, ΔG, for this reaction varies with temperature as shown in. G / kJT / K Δ mol− 1 298 −592.5 288 −594.2 273 −596.7 260 −598.8 240 −602.2 Use these data to plot a graph of free-energy change against temperature on the grid below. Calculate the gradient of the line on your graph and hence calculate the PDF 4. Energy Levels - MIT OpenCourseWare The potential is non-zero and equal to −V H in the region −a ≤ x ≤ a. For a quantum mechanical particle we want instead to solve the Schrodinger equation. We consider two cases. In the first case, the kinetic energy is always positive: −. 1 2 d ψ(x) 2 2m dx 2 = Eψ(x) in Region I − 1 2H. d ψ(x) 2m dx. 22 = (E + V. H
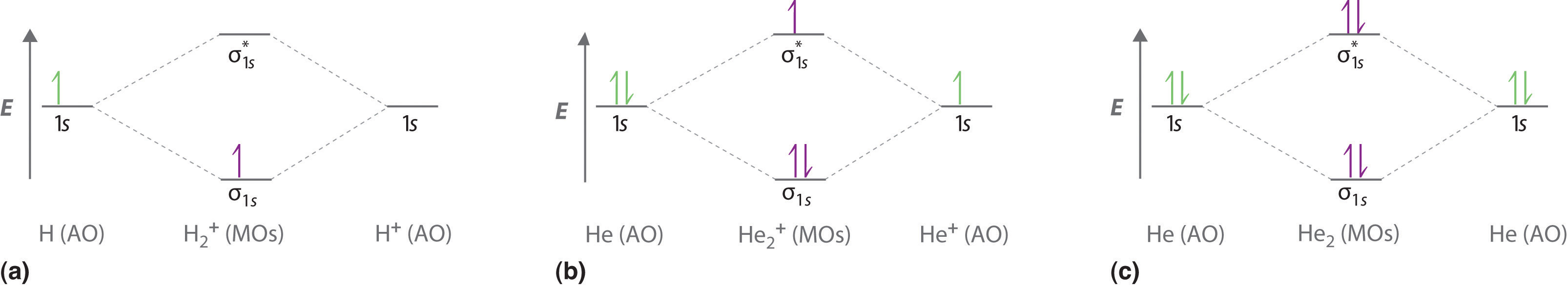
He2 2+ Molecular Orbital Diagram - schematron.org H−. A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining ...
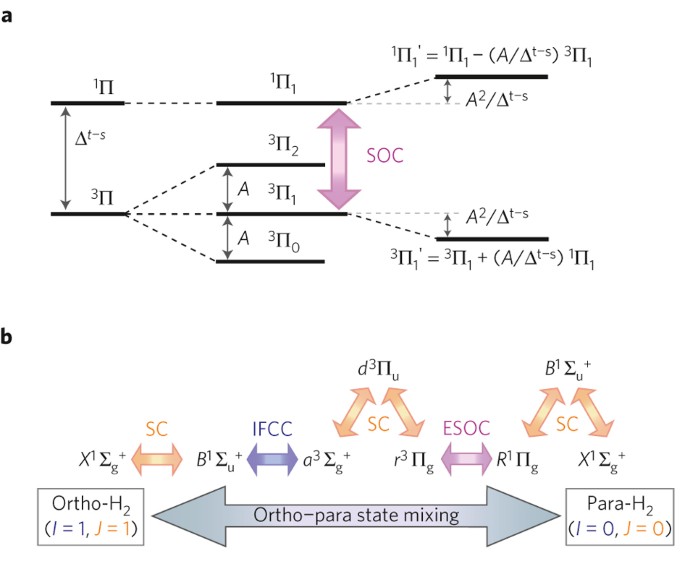
Complete the energy-level diagram for h2−.
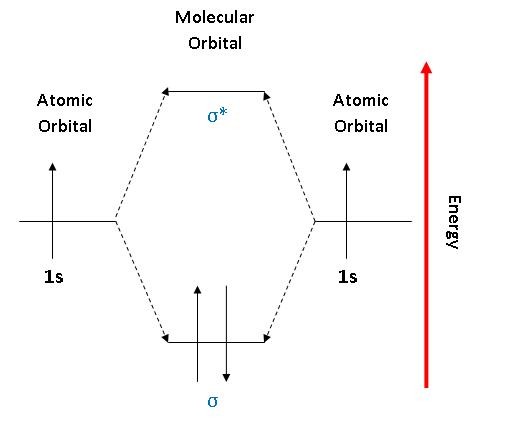
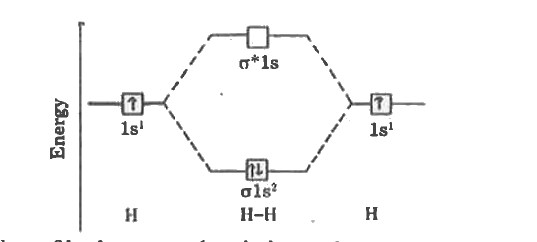
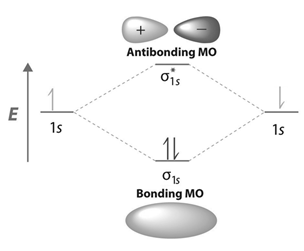
Energy level diagram for Molecular orbitals - Chemical ... N b = 2 , Na =0. Bond order = 1. Positive value of bond order indicates that H 2 molecule is stable.. Bond order value of 1 means that two hydrogen atoms are connected by a single bond.. Greater value of bond order for H 2 molecule than H 2 + ion shows that two H 2 molecule is more stable than H 2 +.. Bond length of H 2 is smaller than that of H 2 + ion.. As no unpaired electron is present ... Molecular Orbital Diagram For He2 - schematron.org A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+.The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the ... Construct The Molecular Orbital Diagram For H2 Chemical bonding - Molecular orbitals of H2 and He2: The procedure can be The molecular orbital energy- level diagram that results is constructed by putting .May 20, · Construct the molecular orbital diagram for H2- and then identify the bond order? i know the answer im just posting it for whoever may need it. (check picture)Status: Open.
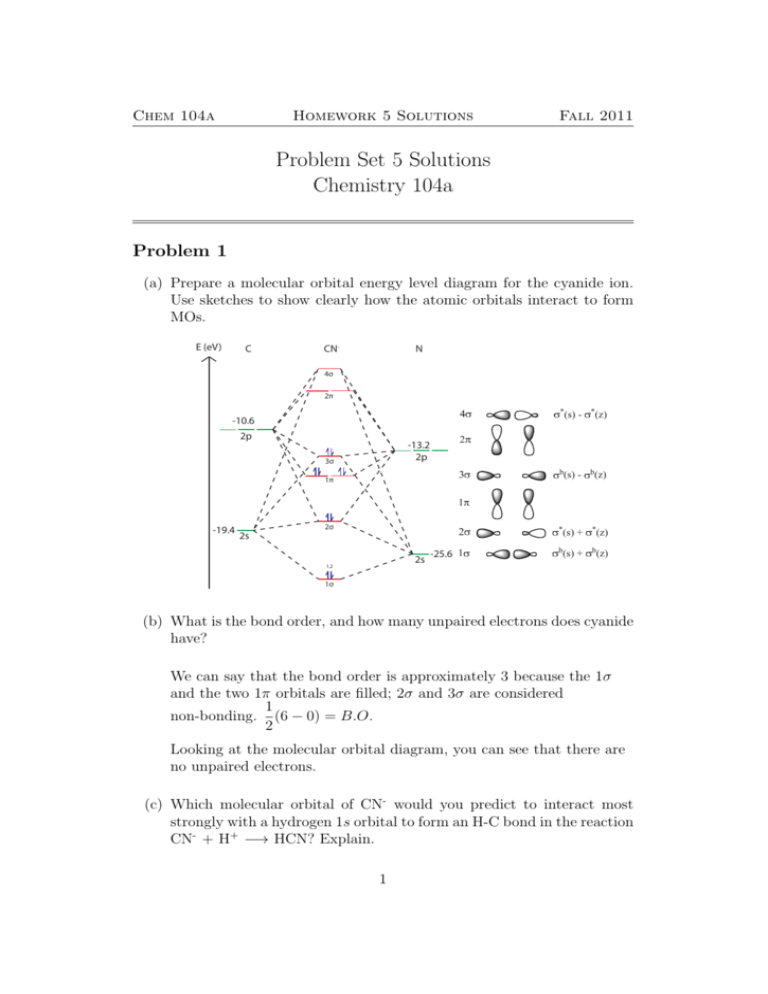
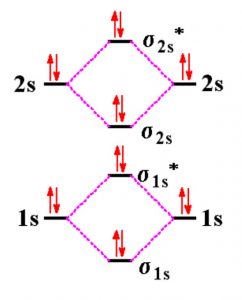
Complete the energy-level diagram for h2−.. CHAPTER 2.0 THERMOCHEMISTRY_NOTES & TUTORIAL Q's - Flip ... Use the data below to construct a Born-Haber cycle (energy level diagram) for copper(II) oxide and determine its lattice energy. Enthalpy of atomisation of copper : +339 kJ mol−1 First ionisation energy of copper : +745 kJ mol−1 Second ionisation energy of copper : +1960 kJ mol−1 Enthalpy of atomisation of oxygen : +248 kJ mol−1 42 complete this molecular orbital diagram for cn - Wiring ... Complete the molecular orbital diagram for CN. Note that the 1s orbitals are not shown. Identify the bond order of CN¯. О 1.5 2p ´2p 2p 2 1 0.5 2.5 3 2s 2s 2.s Answer Bank 11 1 The atomic orbitals on the left side of the molecular The atomic orbitals on the right side of the molecular orbital diagram are those of orbital diagram are those of 9. 8.0 thermochemistry (student's copy) - SlideShare Energy level diagram of Born-Energy level diagram of Born- Haber cycleHaber cycle In the Born-Haber cycle energy diagram, by convention, positive values are denoted as going upwards, negative values as going downwards. 88 89. Example :Example : Given; i. Enthalpy of formation NaCl = - 411 kJmol-1 ii. Molecular Orbital Diagram Be2 - schematron.org Answer to Draw an MO energy diagram and predict the bond order of Be2+ and Be2−. Do you expect these molecules to exist in the. From the above MO diagram we can see that number of elctrons in the bonding and antibonding orbital is same and hence Be does not form Be2 molecule(for.
Be2 Molecular Orbital Diagram - schematron.org Answer to Draw an MO energy diagram and predict the bond order of Be2+ and Be2−. Do you expect these molecules to exist in the. Even rather simple molecular orbital (MO) theory can be used to predict which we start reading from the bottom of the diagram because this is how MO diagrams are constructed, Diberyllium, Be2, has a bond order of zero and is unknown. DOCX Quia (a) Carefully complete the energy diagram by drawing a curve that accurately shows the progress of the reaction, beginning at the reactants, moving through the transition state, and ending at the final products. When adding the products to the diagram, be sure to use an energy level that is appropriate relative to the energy level of the reactants. PDF The John Fisher School Summer revision calculations for AS ... The C −Br bond is weaker than the C−Cl bond. The H −Cl bond is weaker than the H−Br bond. Chlorine is more reactive than bromine. (1) (Total 15 marks) Q3.A student investigated the rate of reaction of magnesium and hydrochloric acid. Mg(s) + 2HCl(aq) MgCl 2 (aq) + H 2 (g) Some species consisting of just two oxygen ... - Clutch Prep You may want to reference (Pages 369 - 371) Sections 9.7 and 9.8 while completing this problem.Complete the energy-level diagram for H2-. Q. You may want to reference (Pages 369 - 371) Sections 9.7 and 9.8 while completing this problem.Sketch the molecular orbitals of the H2- ion and draw...
CHEM 123 SAPLING LEARNING CHAPTER 14 Flashcards - Quizlet CHEM 123 SAPLING LEARNING CHAPTER 14. The rate of a reaction is studied by measuring the concentration of the reactant and the concentration of the product as a function of time. The results are shown in the graph. A graph with time on the x axis and concentration on the y axis. A red curve labeled X, starts at a concentration of zero at time zero. PDF Miessler-Fischer-Tarr5e SM Ch 05 CM The energy level diagram for CO2 in Figure 5.25 can be used as a guide, with the orbitals of Be higher in energy than those of C and the orbitals of F lower in energy than those of O. Calculated molecular orbital shapes are below, for comparison for those of CO2 in Figure 5.25. chem 1201 HW Ch. 6 p 2 Flashcards | Quizlet chem 1201 HW Ch. 6 p 2. Indicate whether energy is emitted or absorbed when the following electronic transitions occur in hydrogen. Using equation E= (hcRH) (1n2)= (−2.18×10−18J) (1n2), calculate the energy of an electron in the hydrogen atom when n=2. Nice work! Molecular Orbital Diagram For He2 A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 .
AP CHEM FINAL Flashcards | Quizlet On the right side of the energy diagram, draw a horizontal line segment to indicate the energy of the products. ... H2(g) 0 A −373kJ/mol−373kJ/mol B −111kJ/mol−111kJ/mol C +111kJ/mol+111kJ/mol D ... In the following box, complete the Lewis electron-dot diagram for a molecule of methylamine. Show all bonding and nonbonding valence electrons.
Draw the partial (valence-level) orbital d... | Clutch Prep Q. Draw the partial (valence-level) orbital diagram, and write the symbol, group number, and period number of the element: (b) [Ar] 4s23d104p3. Q. Draw a partial (valence-level) orbital diagram, and write the condensed ground-state electron configuration for each: (c) Ag.
PDF Quantum Physics III Chapter 2: Hydrogen Fine Structure and the rest energy of the electron is mc2. Then, e2 a 0 = me4 ~2 = mα2~2c2 ~2 = α2 mc2. (2.1.5) This states that the energy scale of hydrogen bound states is a factor of α2 smaller than the rest energy of the electron, that is, about 19000 times smaller. We can thus rewrite the possible energies as: En = −1 2α 2 mc2 1 n. (2.1.6)
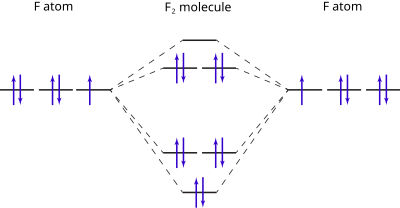
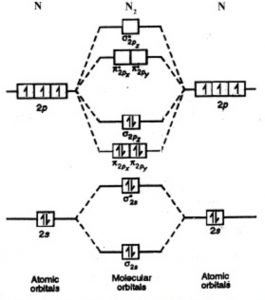
Draw the molecular orbital energy level diagram of N2 ... With the help of energy levels homonuclear diatomic orbitals, arrange the following species in increasing order of stability O 22−. +. Draw molecular orbital diagram of O 2 or N 2 with magnetic behavior and bond order. Use the molecular orbital energy level diagram to show that N 2 would be expected to have a triple bond, F 2 , a single bond ...
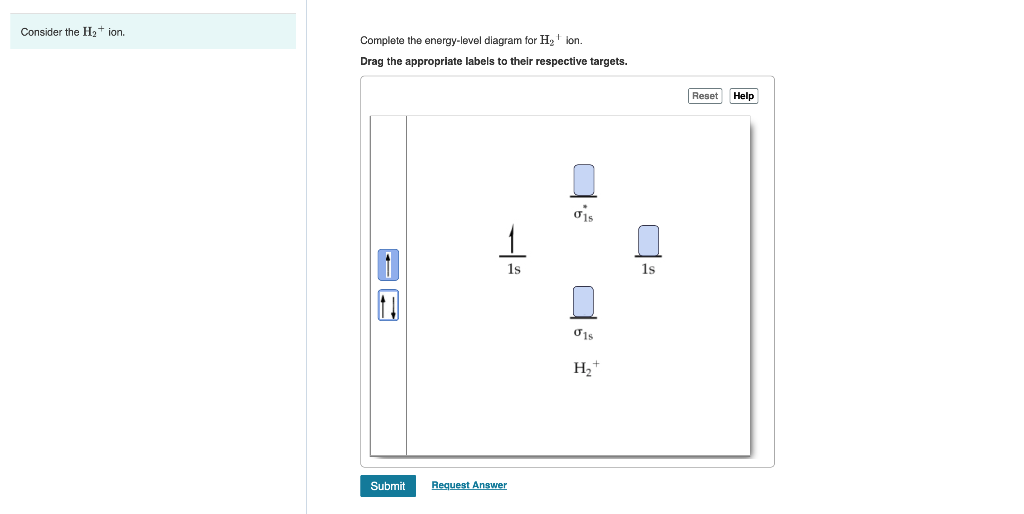
Solved Complete the energy-level diagram for H2 Drag the ... Chemistry. Chemistry questions and answers. Complete the energy-level diagram for H2 Drag the appropriate labels to their respective targets Reset Help σ1s 1s 1s 14 1s H2.
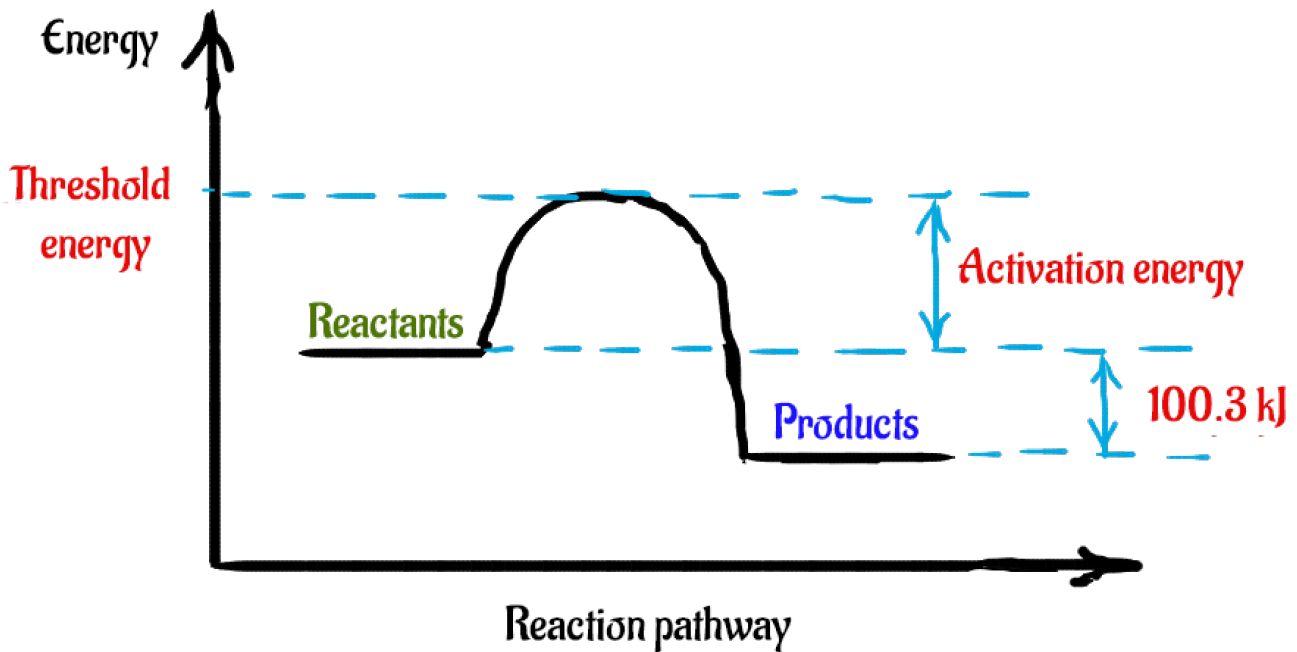
OCR - Module 3 Topic Test Q14 - The Student Room H2 (g) + I2 (g) ⇌ 2HI (g) The activation energy for the forward reaction is 173 kJ mol-1. (a) Complete the enthalpy profile diagram below for the forward reaction in equilibrium 3.1. On your diagram: • Label the activation energy, Ea. • Label the enthalpy change of reaction, DH.
Enthalpy,energy,rate of reaction Flashcards - Quizlet Hydrogen iodide, HI, is a colourless gas that can be made from the reaction of hydrogen, H2, and equilibrium 3.1 iodine, I2. This reversible reaction is shown in equilibrium 3.1 below. The activation energy for the forward reaction is 173 kJ mol−1. (a) Complete the enthalpy profile diagram below for the forward reaction in equilibrium 3.1.
He2 2+ Molecular Orbital Diagram - Wiring Diagrams H−. Bond order = 1. 2 (electrons in bonding orbitals - electrons in antibonding orbitals) Draw a complete MO diagram for all the bonds in ethene.The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule.
Construct The Molecular Orbital Diagram For H2 Chemical bonding - Molecular orbitals of H2 and He2: The procedure can be The molecular orbital energy- level diagram that results is constructed by putting .May 20, · Construct the molecular orbital diagram for H2- and then identify the bond order? i know the answer im just posting it for whoever may need it. (check picture)Status: Open.
Molecular Orbital Diagram For He2 - schematron.org A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+.The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the ...
Energy level diagram for Molecular orbitals - Chemical ... N b = 2 , Na =0. Bond order = 1. Positive value of bond order indicates that H 2 molecule is stable.. Bond order value of 1 means that two hydrogen atoms are connected by a single bond.. Greater value of bond order for H 2 molecule than H 2 + ion shows that two H 2 molecule is more stable than H 2 +.. Bond length of H 2 is smaller than that of H 2 + ion.. As no unpaired electron is present ...







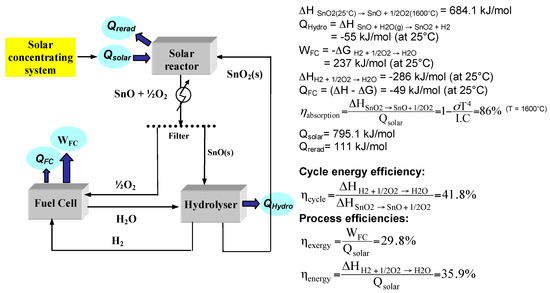


















0 Response to "42 complete the energy-level diagram for h2−."
Post a Comment