43 molecular orbital diagram h2
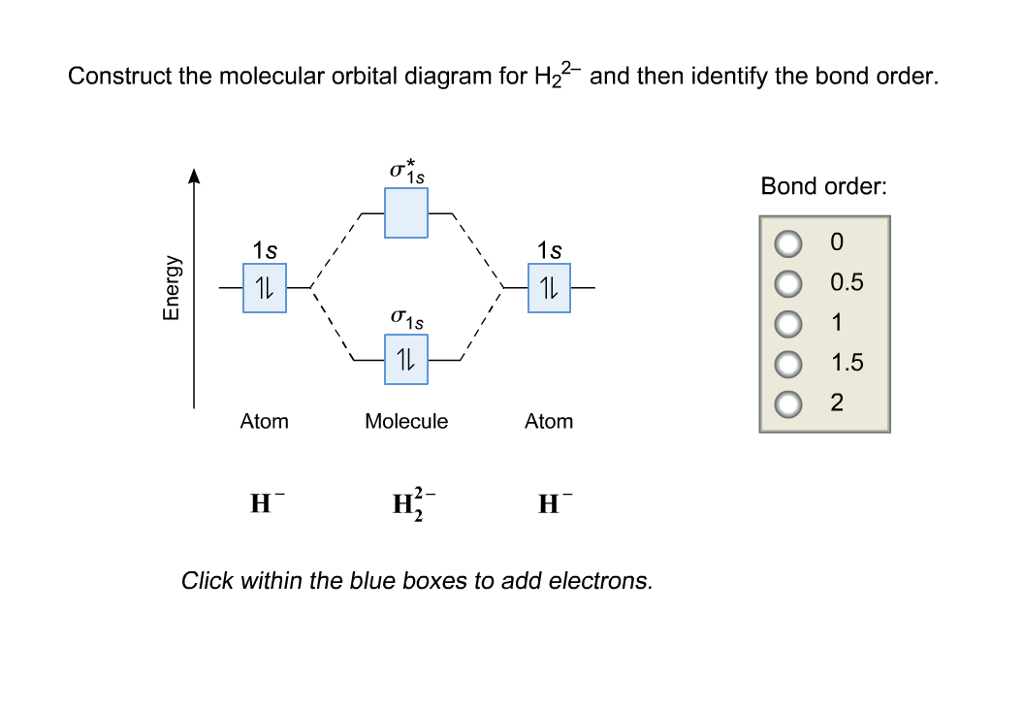
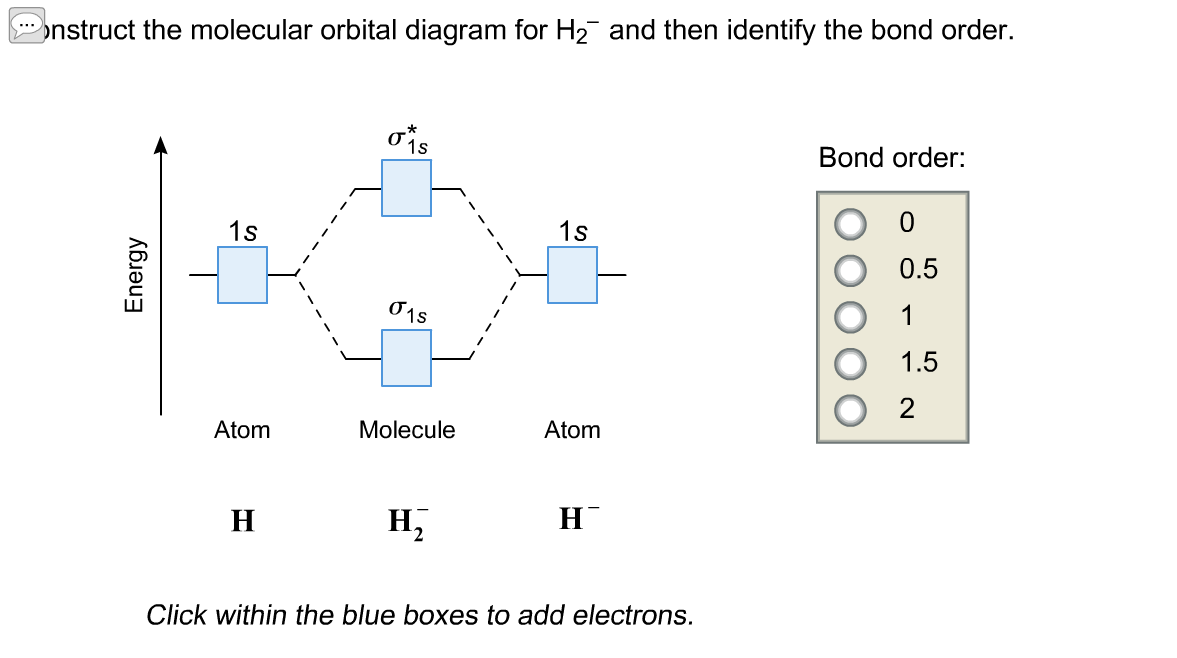
Construct the molecular orbital diagram fo... | Clutch Prep Problem: Construct the molecular orbital diagram for H2- and then identify the bond order. Click thin the blue boxes to add electrons.Bond order: a) 0 b) 0.5c) 1 d) 1.5e) 2 Molecular Orbitals - Introductory Chemistry - 1st Canadian ... However, the molecular orbital diagram we see in Figure 9.25 ("Molecular orbital energy diagram for homonuclear diatomic molecules made from atoms of atomic number 5-7") can be used to estimate the electron configuration and bond order. Frontier Molecular Orbitals.
39 h2+ molecular orbital diagram - Diagram Online Source 39 h2+ molecular orbital diagram. In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule.This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region.
Molecular orbital diagram h2
PDF 1 Lecture 2 Simple Molecular Orbitals - Sigma and Pi Bonds ... LUMO = lowest unoccupied molecular orbital HOMO = highest occupied molecular orbital Similar phase of electron density (no node) adds together constructively. energy of isolated atoms bond order (H2 molecule) = (2) - (0) 2 = 1 bond 1sb H H H H σ∗ = 1s H H a - 1sb = antibonding MO = LCAO = linear combination of atomic orbitals node = zero ... How do I calculate the bond order for H2- and H2+? | Socratic Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, H− 2 has three electrons while H+ 2 has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one σ1s and one σ* 1s MO by conservation of orbitals. How to Make the Molecular Orbital Diagram for H2(2+): Does ... This video discusses how to draw the molecular orbital (MO) diagram for the H2(2+) molecule. The bond order of H2(2+) is calculated and the meaning of this n...
Molecular orbital diagram h2. PDF Molecular Orbital (MO) Theory of the H2 molecule Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic ... Spin‐orbitals of type 1 and 3 have the same symmetry, and therefore can "mix" (to give improved wavefunctions and energy eigenvalues): 1 ψψ αβ ... Construct The Molecular Orbital Diagram For H2 And Then ... You are watching: Construct the molecular orbital diagram for h2 and then identify the bond order A molecular orbital diagram is used to define chemical bonding in a molecule. This chart is based ~ above the molecular orbital theory. 43 construct the molecular orbital diagram for h2 below ... Construct the molecular orbital diagram for h2 below. What is the molecular orbital diagram for B_2? | Socratic The video below describes how to generate molecular orbital diagrams for B₂ and other diatomic molecules from Row 2 elements of the Periodic Table. PDF Chapter 6 - Molecular Structure In the molecular representations below, the ... Molecular orbital diagram of h2 - fornoob.com Molecular orbital diagram of h2 Answer General guidance Concepts and reason The bonding and anti - bonding interaction of the molecules can be explained with the help of a molecular orbital diagram. It also gives a detailed description of bonding in molecules. Bond order represents the number of bonds present in between two bonded atoms.
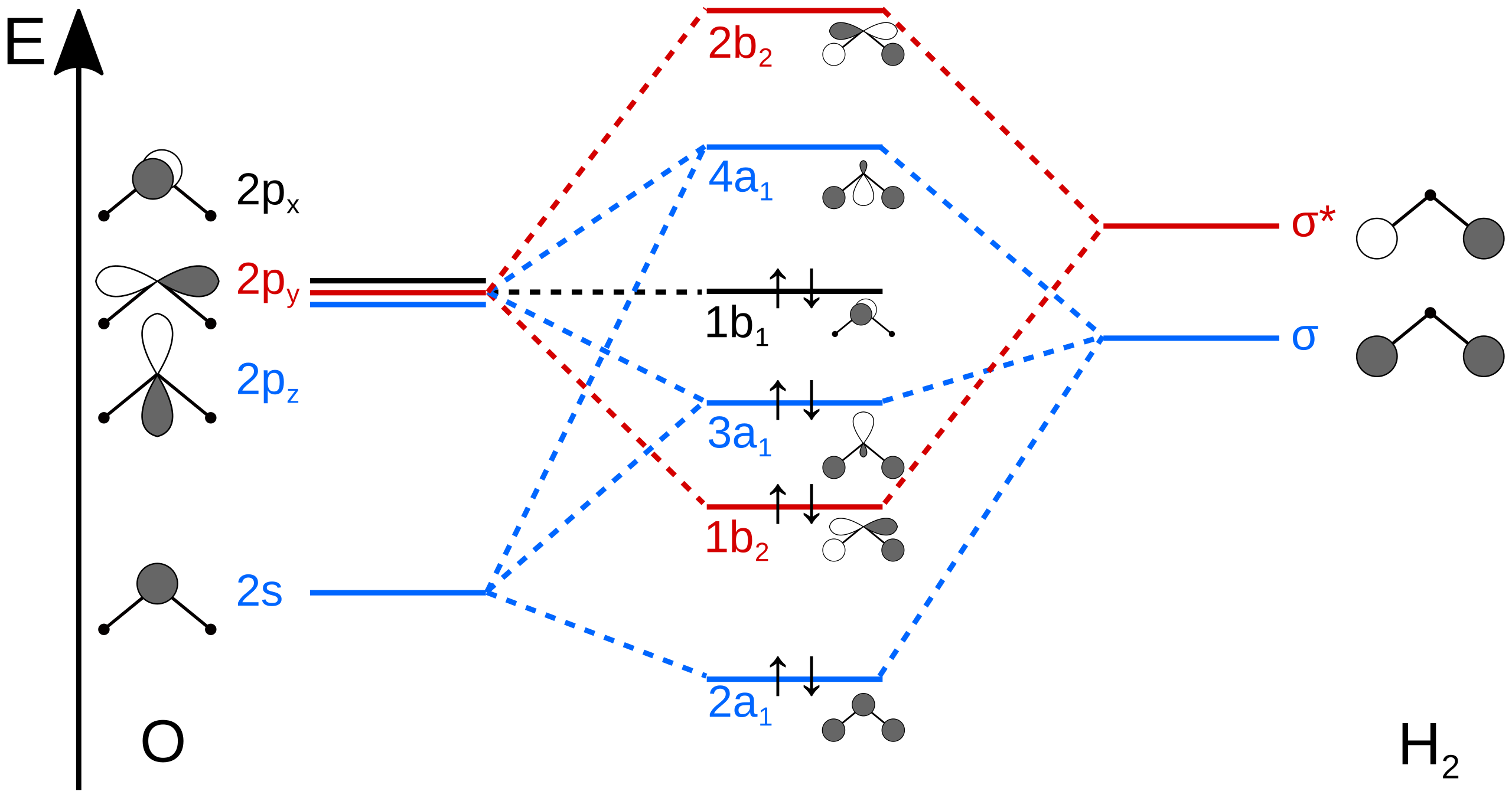
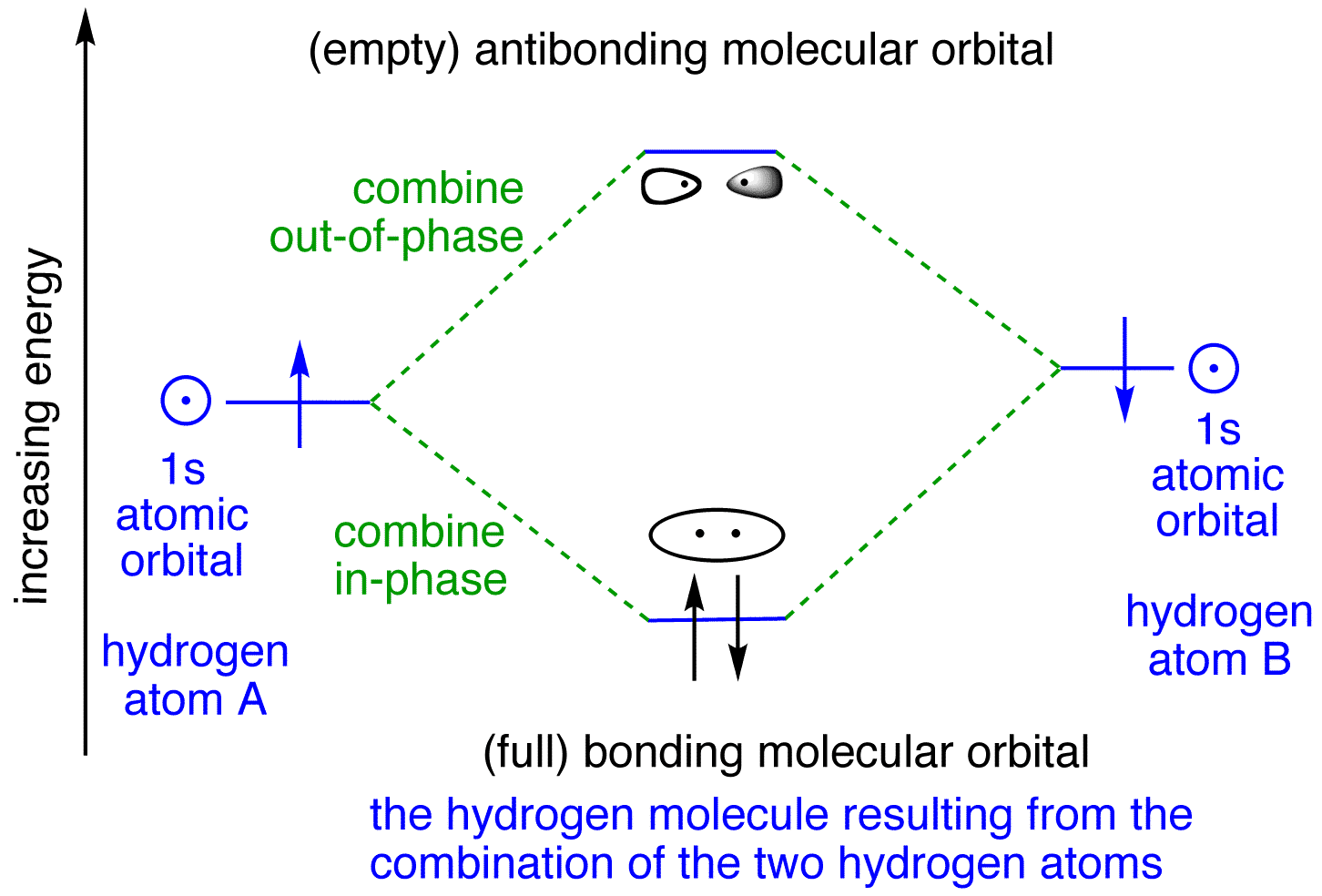
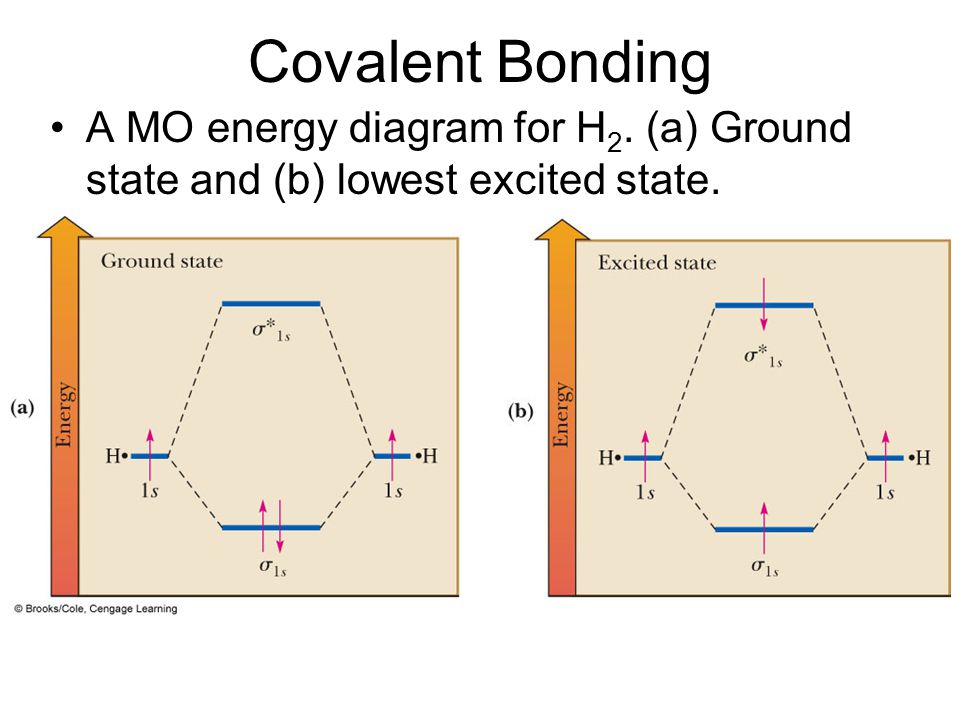
Draw a molecular orbital diagram of N2 or O2 with magnetic ... Draw a molecular orbital diagram of ${N_2}$ or ${O_2}$ with magnetic behavior and bond order. Verified. 92.9k+ views. Hint: Generally the molecular orbital diagrams are used to understand the bonding of a diatomic molecule. You should know that molecular orbital diagrams are used to deduce magnetic properties of a molecule; they also help us to ... Solved Construct the molecular orbital diagram for H2- and ... Solved Construct the molecular orbital diagram for H2- and | Chegg.com. Science. Chemistry. Chemistry questions and answers. Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes to add electrons. Question: Construct the molecular orbital diagram for H2- and then identify the ... chemical bonding - Molecular orbitals of H2 and He2 ... The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure 13. He2 2+ Molecular Orbital Diagram - schematron.org the molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the h 2 molecule is shown in figure on either side of the central ladder are shown the energies of the 1 s orbitals of atoms a and b, and the central two-rung ladder shows the energies of the bonding and antibonding.the …
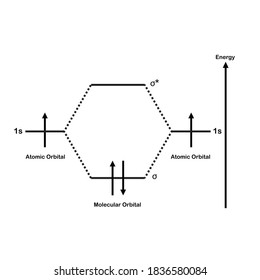
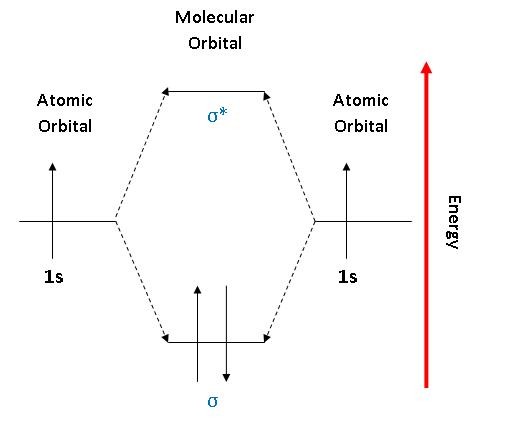
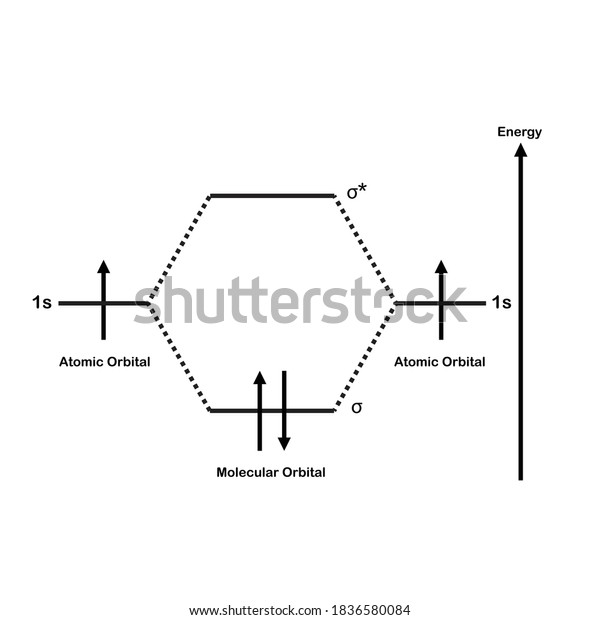
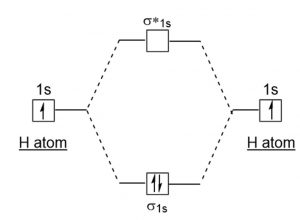
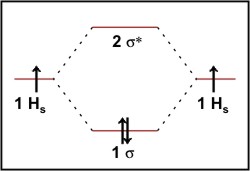
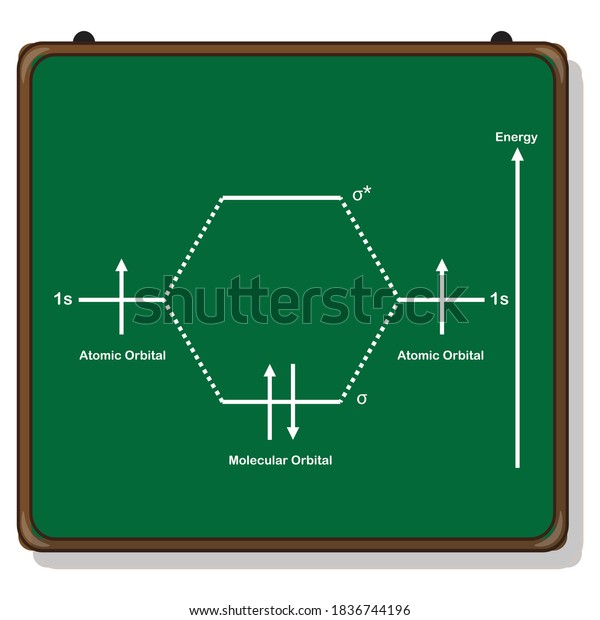
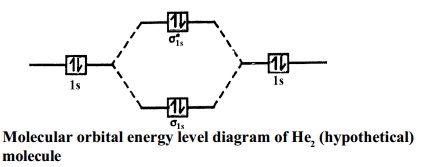
Molecular Orbital Diagram For He2 A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 . Molecular Orbital Diagram Of H2 - schemacheck.com Molecular Orbital Diagram Of H2 For the diatomic molecules like hydrogen and helium, the following molecular orbital diagram is used. antibonding molecular orbital Energy - Is. For H2, bond order = 1/2 () = 1, which means H2has only one bond. The antibonding orbital is empty. Thus, H2 is a stable molecule. Molecular Orbital Theory - Chemistry Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds. Construct The Molecular Orbital Diagram For H2 Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here.
Is H2 a viable molecule for the molecular orbital ... - Quora Answer (1 of 2): According to the molecular orbital theory, a molecular is viable of its bond order is more than or equal to one. The bond order is defined as number of bond between to two atoms of that molecule. It is calculated as the difference of electrons in bonding molecules and anti-bondin...
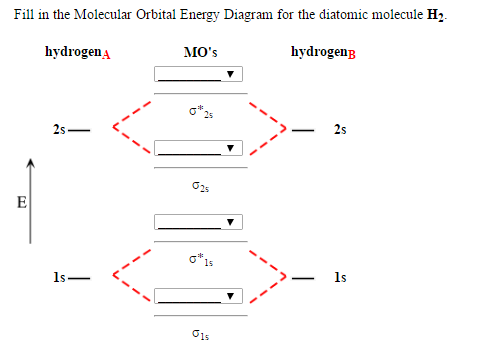
6. Draw the molecular orbital energy level diagram of H2 Draw the molecular orbital energy level diagram of H2. Answer. Prev Question Next Question. Related Questions to study. The degenerate orbitals of ... The metal d-orbitals that are directly facing the ligands in K 3 ...
Molecular Orbital (MO) Diagram of H2 - YouTube Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me ...
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
Molecular Orbital Theory - Purdue University starting with the lowest energy molecular orbital. The two electrons associated with a pair of hydrogen atoms are placed in the lowest energy, or bonding, molecular orbital, as shown in the figure below. This diagram suggests that the energy of an H2molecule is As a result, the H2molecule is more stable than a pair of isolated atoms.
Energy level diagram for Molecular orbitals - Chemical ... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ...
PDF Simple Molecular Orbital Theory - University of California ... is called Molecular Orbital Theory. • MO theory assumes that the valence electrons of the atoms within a molecule become the valence electrons of the entire molecule. • Molecular orbitals are constructed by taking linear combinations of the valence orbitals of atoms within the molecule. For example, consider H2:
Complete An Mo Energy Diagram For H2+. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.The Hydrogen Molecule Ion H2+Molecular Orbital Diagrams of Diatomic Molecules - Chem
How to Make the Molecular Orbital Diagram for H2(2+): Does ... This video discusses how to draw the molecular orbital (MO) diagram for the H2(2+) molecule. The bond order of H2(2+) is calculated and the meaning of this n...
How do I calculate the bond order for H2- and H2+? | Socratic Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, H− 2 has three electrons while H+ 2 has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one σ1s and one σ* 1s MO by conservation of orbitals.
PDF 1 Lecture 2 Simple Molecular Orbitals - Sigma and Pi Bonds ... LUMO = lowest unoccupied molecular orbital HOMO = highest occupied molecular orbital Similar phase of electron density (no node) adds together constructively. energy of isolated atoms bond order (H2 molecule) = (2) - (0) 2 = 1 bond 1sb H H H H σ∗ = 1s H H a - 1sb = antibonding MO = LCAO = linear combination of atomic orbitals node = zero ...




















0 Response to "43 molecular orbital diagram h2"
Post a Comment