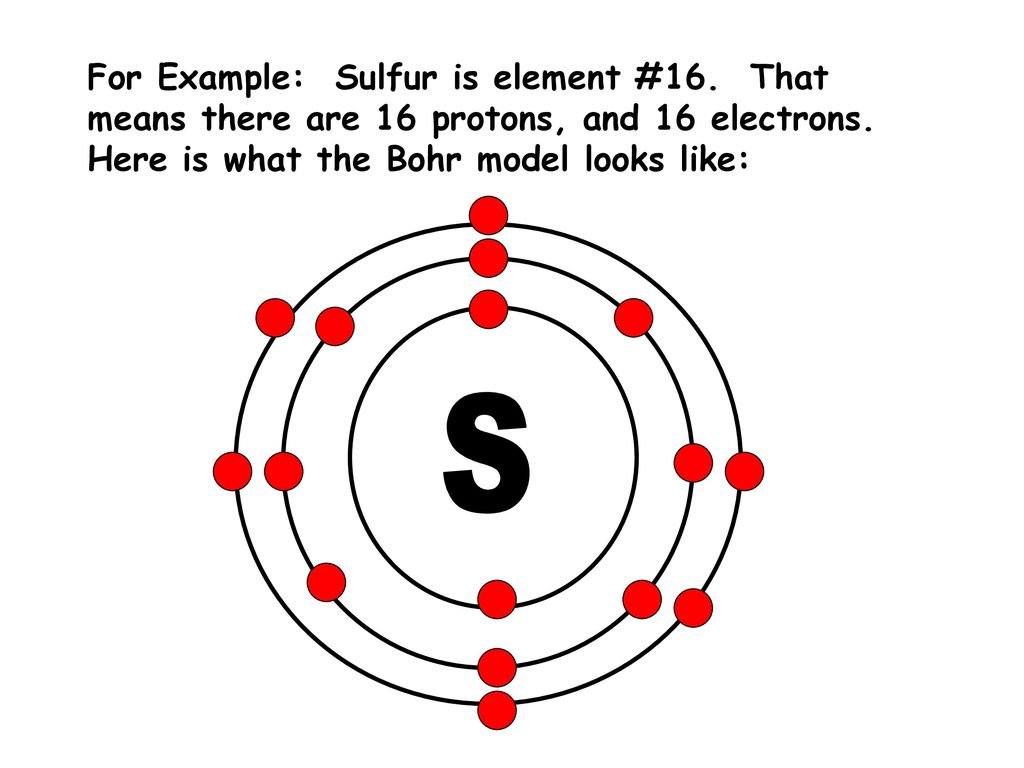
44 lewis dot diagram for sulfur
Also asked, what is the Lewis dot structure for sulfur? Now let us try Lewis dot structure of Sulfide ion ( S 2-). Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). Lewis dot structure will have 4 paired dots around Sulfur atom. (15) Lewis Diagrams Obj. 15. From the name of a molecular chemical, determine the Lewis (electron dot) diagram for it.. In these cases, you know that you are dealing with nonmetal atoms bonded together with covalent bonding and that (some of) the valence electrons of the atoms are shared between the atoms.
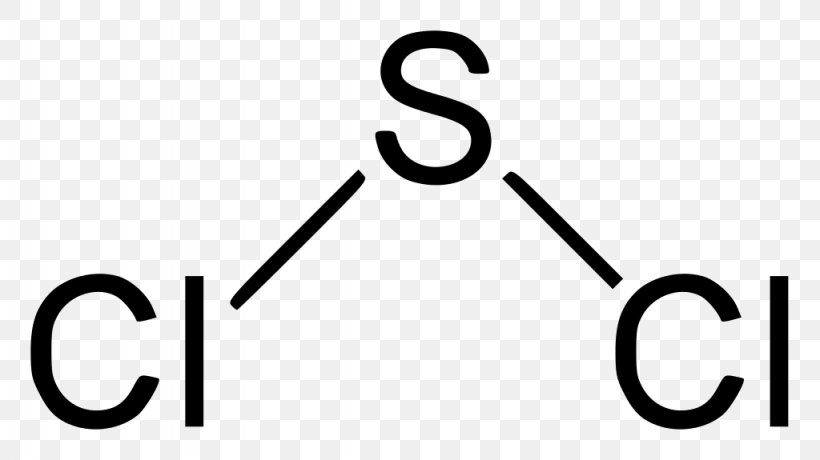
The. Question: Draw the Lewis-dot structure for sulfur dichloride, SC12. Which statement best describes the correct Lewis-dot structure. Sulfur is the center atom that is double bonded to the chlorine atoms. The sulfur atom has 4 lone pairs of electron while each chlorine atom has four lone pairs. Sulfur is the center atom.

Lewis dot diagram for sulfur
What is the correct electron dot diagram for a neutral atom of SULFUR ? Answer : Surfum (s) electronic configuration, is? 252 286 352 3p 4 valence shell for Sulfur 352 384 The ne fone, electron dot diagram of a neutral sulfur is. :5: Hottest videos. Thereof, what is the Lewis dot structure for sulfur? Now let us try Lewis dot structure of Sulfide ion ( S 2-). Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). Lewis dot structure will have 4 paired dots around Sulfur atom. Lewis dot structure for cas. October 22, 2021 thanh. 2. Draw the Lewis structures for the formation of CaS from the calcium and sulfur atoms (3 pts) 2.
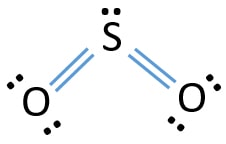
Lewis dot diagram for sulfur. Sulfur (S) has six valence electrons. A Lewis dot structure of around 'S' would have two dots on two sides, and one single on each of the remaining. It would look be drawn as: .. .S : . After that I draw the Lewis dot structure for Sulfur (S). Note: Sulfur is in Group 16 (sometimes called Group VI or 6A). Since it is in Group 6 it will have 6 valence electrons. When you draw the Lewis structure for Sulfur you'll put six "dots" or valance electrons around the element symbol (S). Explore further detail here. Jan 31, 2022 · Lewis structure of SO3 The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation. The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access . Drawing the Lewis Structure for SO 2.
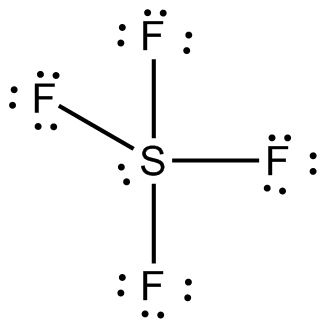
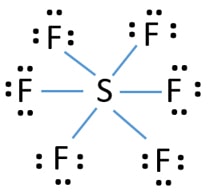
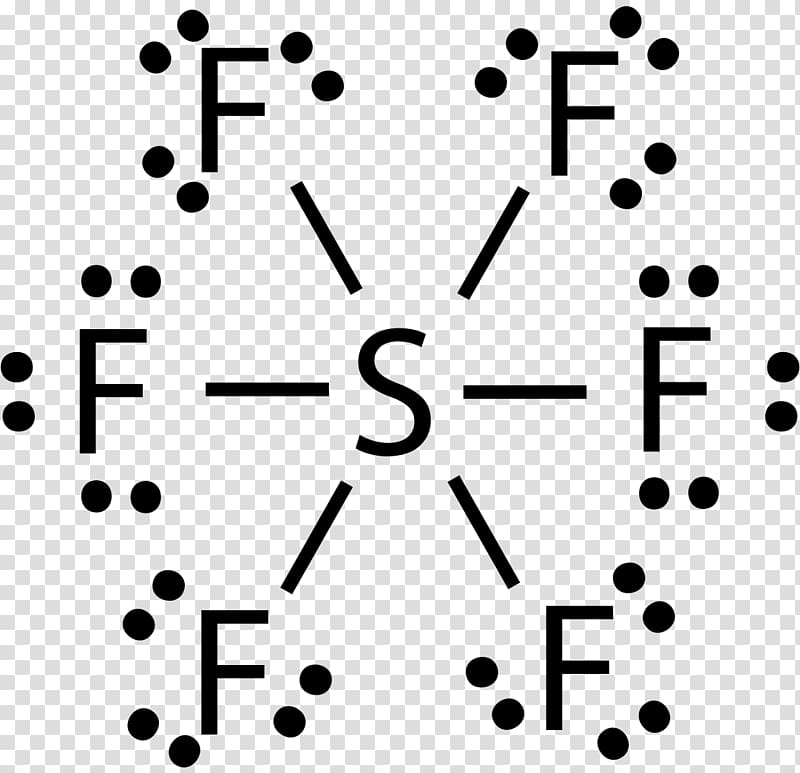
The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access to the . Here are the steps I follow when drawing a Lewis structure. SF 6 (Sulfur hexafluoride) Lewis Structure. SF 6 (Sulfur hexafluoride) molecule contains one sulfur atom and six fluorine atoms. Lewis structure of SF 6 is given below. In SF 6 lewis structure, each fluorine atom has made single bonds with center sulfur atom. There are no lone pairs on sulfur atom and three lone pairs on each fluorine atom. In this tutorial, we will learn how to draw the lewis ... Lewis dot structure of Nitride ion. Now let us try Lewis dot structure of Sulfide ion ( S 2-).Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). [Ne]4s 2 4p 6. Valence electrons are 8 (2 in 3s and 6 in 3p) Lewis dot structure of sulfide ion A step-by-step explanation of how to draw the Lewis dot structure for S (Sulfur). I show you where Sulfur is on the periodic table and how to determine how ...
Drawing the Lewis Structure for SO 3 ( Sulfur Trioxide) SO 3 is the primary contributer to acid rain in the atomsphere. It is a form of pollution. SO 3 is named Sulfur Trioxide. There are 32 valence electrons available for the Lewis structure for SO 3. Be sure to check the formal charges for the Lewis structure for SO 3 . To sketch the SBr2 Lewis structure by following these instructions: Step-1: SBr2 Lewis dot Structure by counting valence electrons on the sulfur atom. Step-2: Lewis Structure of SBr2 for counting valence electrons around the terminal bromine atoms. Step-3: Lewis dot Structure for SBr2 generated from step-1 and step-2. Lewis Structure Bromine Pentafluoride Sulfur Tetrafluoride Xenon. View the full answer. lewis structure sbr6 dot diagram draw o2 carbon monoxide sulfur hexabromide tetraiodide compounds ci4. This gives you 5 regions of electron density around the central atom. Lewis Structure Sketch of Shape I need to do the above things for SBr6 and BrCl5. For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4s 2 3d 6) is as follows:. Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration.
Lewis structure for sbr2 Lewis dot structure for sbr2. The chemical fury dibromide of sulfur is SBR2. Drawing SBR2 Lewis structure is very easy to use the following month. Here in this post, we describe the method step by step to build the structure of SBR2 Lewis.
Lewis Dot Structure For Sulfur Dichloride, SCl2 Lewis Structure: How to Draw the Lewis Structure for, hexanedioyl dichloride 111 50 2, C6H8Cl2O2, density, Chemistry Chemical Bonding (9 of 35) Lewis Structures, VSEPR Theory: Valence Shell Electron Pair Repulsion Theory
Lewis Dot Diagram For Copper - 9 images - diagram lewis dot diagram for ionic compound full, 31 sulfur atom diagram wiring diagram database,
A Lewis electron dot diagram A representation of the valence electrons of an atom that uses dots around the symbol of the element. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
Lewis Dot Diagram For So4 2. Simple procedure for drawing covalent Lewis structures - Lewis dot of the sulfate ion SO, best lewis structure for so, electron bonding. Viewing Notes: The Lewis structure for SO is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis.
Follow some steps for drawing the lewis dot structure for SBr2. 1. Count total valence electron in SBr2. In the very first step, we have to count the number of valence electrons available for drawing the lewis structure of SBr2. For this, we have to count the valence electrons in sulfur and bromine atoms.
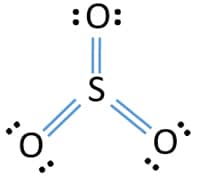
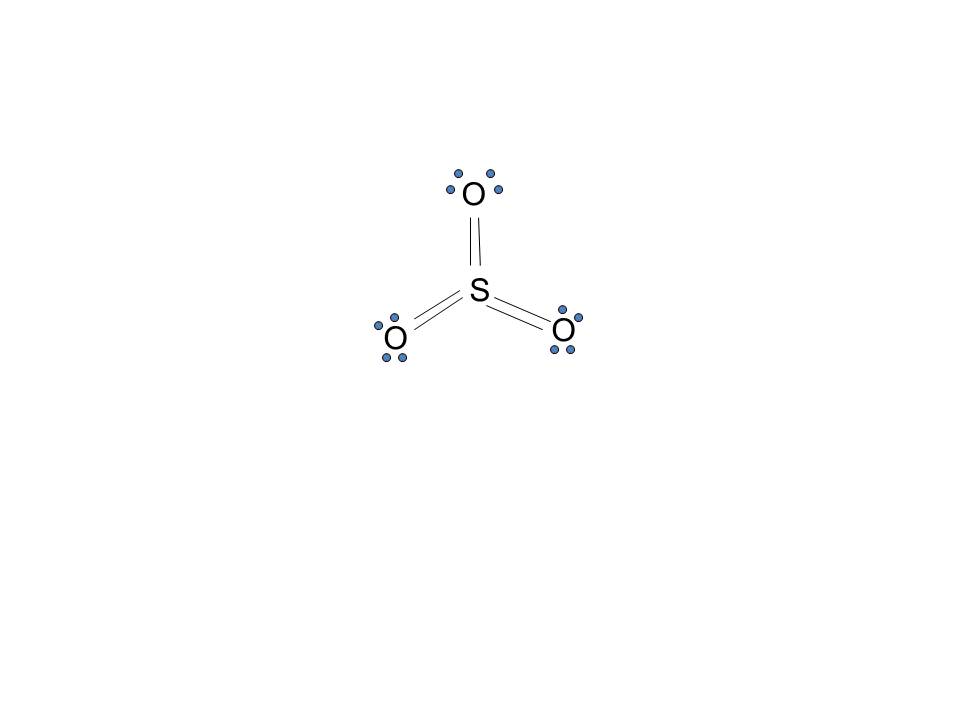
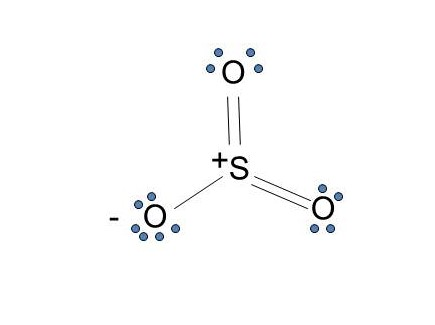
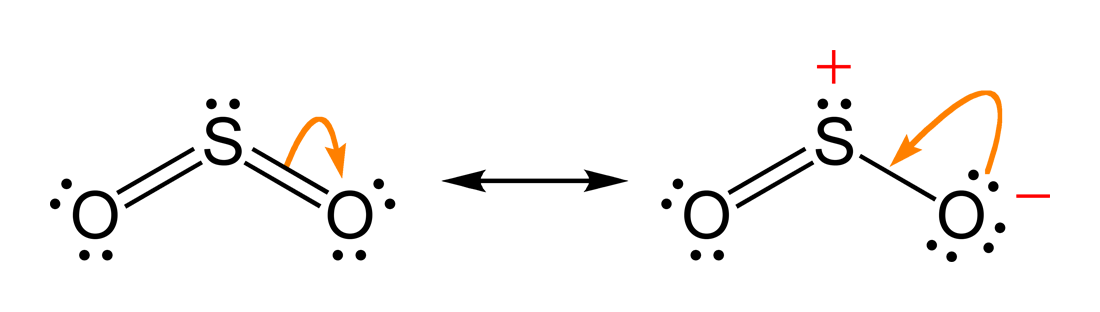
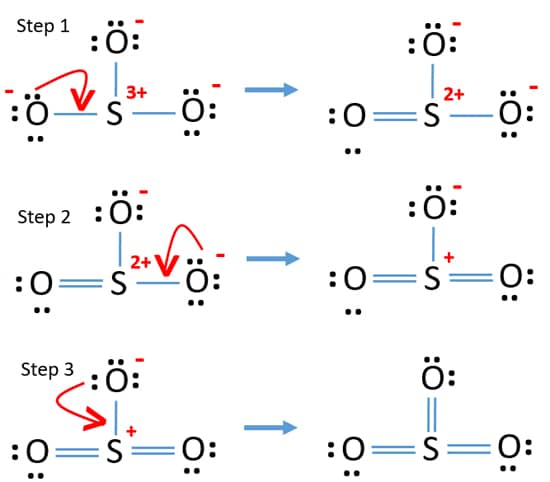
The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. And then we have a lone pair of electrons on our sulfur.
Lewis dot structure for cas. October 22, 2021 thanh. 2. Draw the Lewis structures for the formation of CaS from the calcium and sulfur atoms (3 pts) 2.
Thereof, what is the Lewis dot structure for sulfur? Now let us try Lewis dot structure of Sulfide ion ( S 2-). Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). Lewis dot structure will have 4 paired dots around Sulfur atom.
What is the correct electron dot diagram for a neutral atom of SULFUR ? Answer : Surfum (s) electronic configuration, is? 252 286 352 3p 4 valence shell for Sulfur 352 384 The ne fone, electron dot diagram of a neutral sulfur is. :5: Hottest videos.









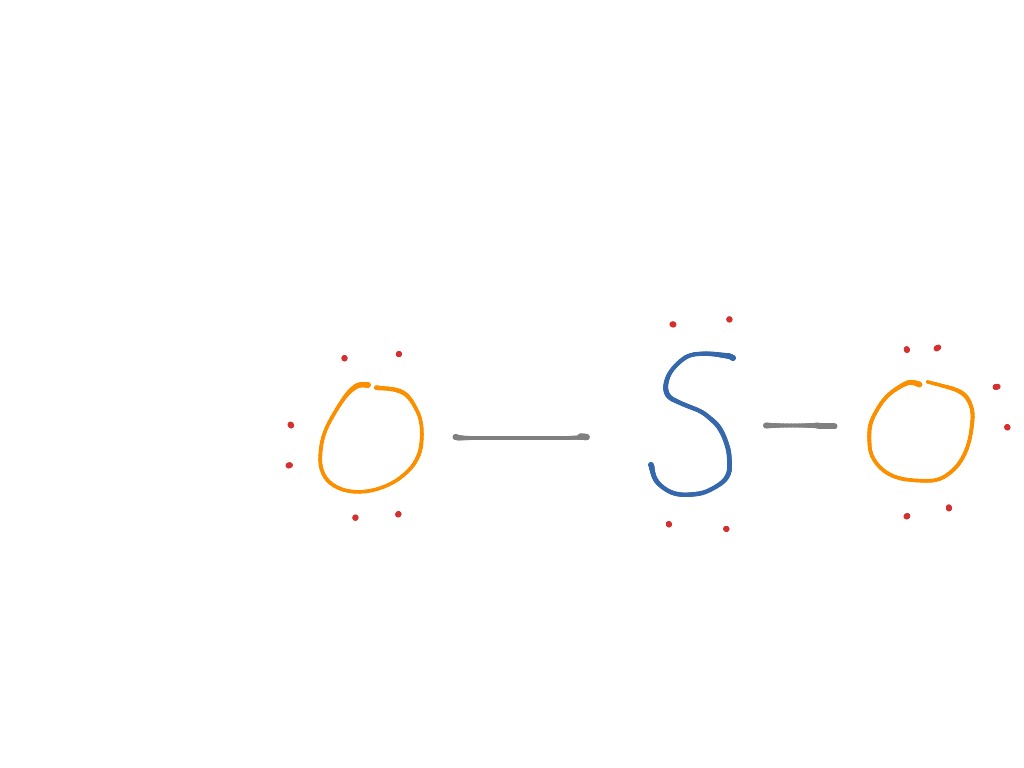



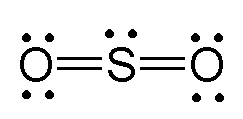












0 Response to "44 lewis dot diagram for sulfur"
Post a Comment