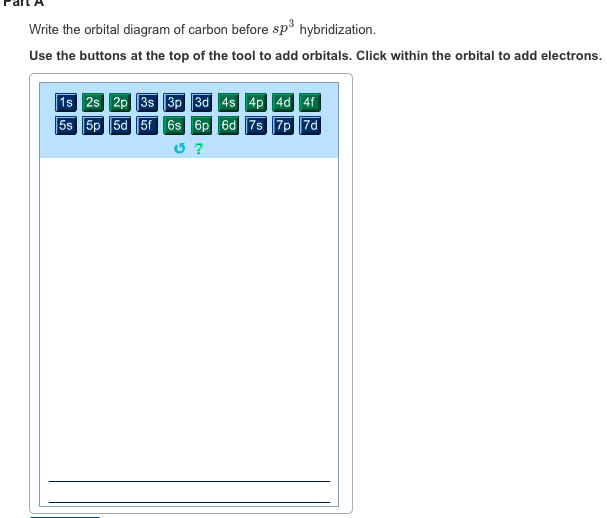
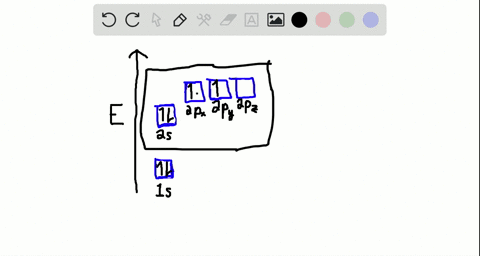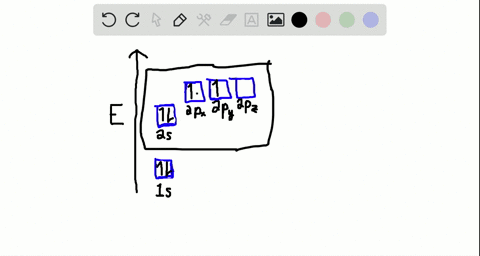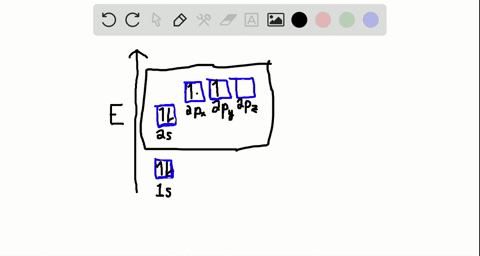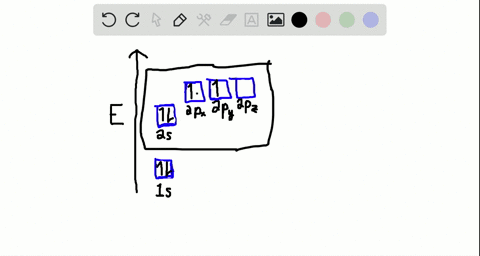
44 write the orbital diagram of carbon before sp3 hybridization
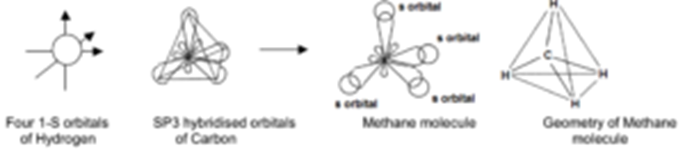
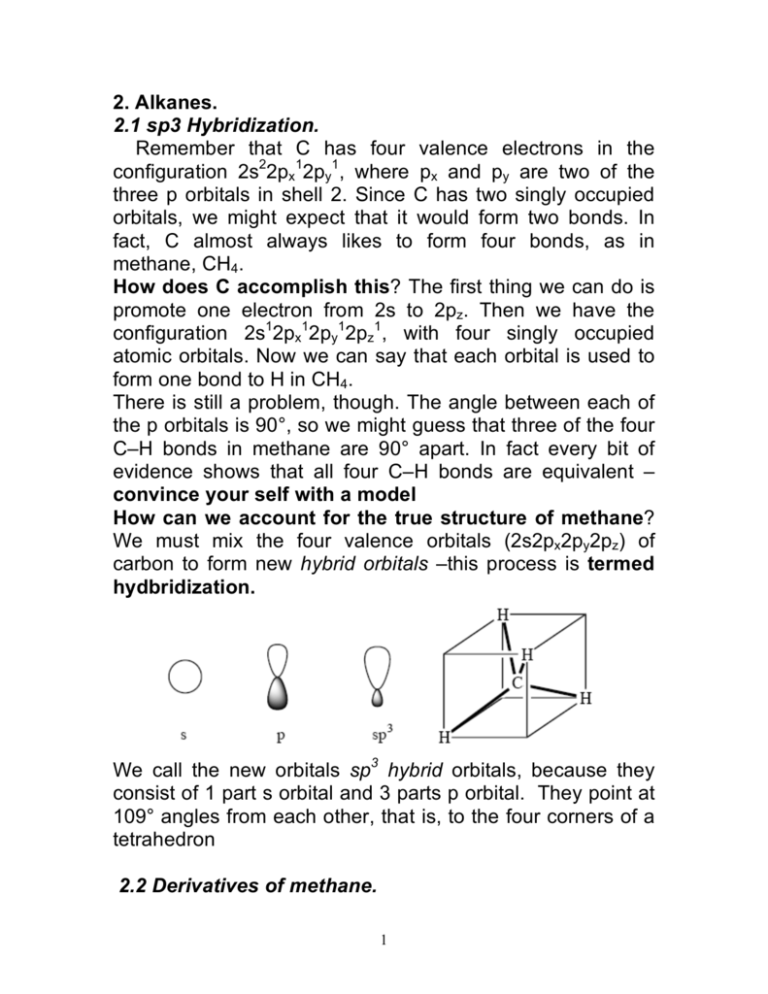
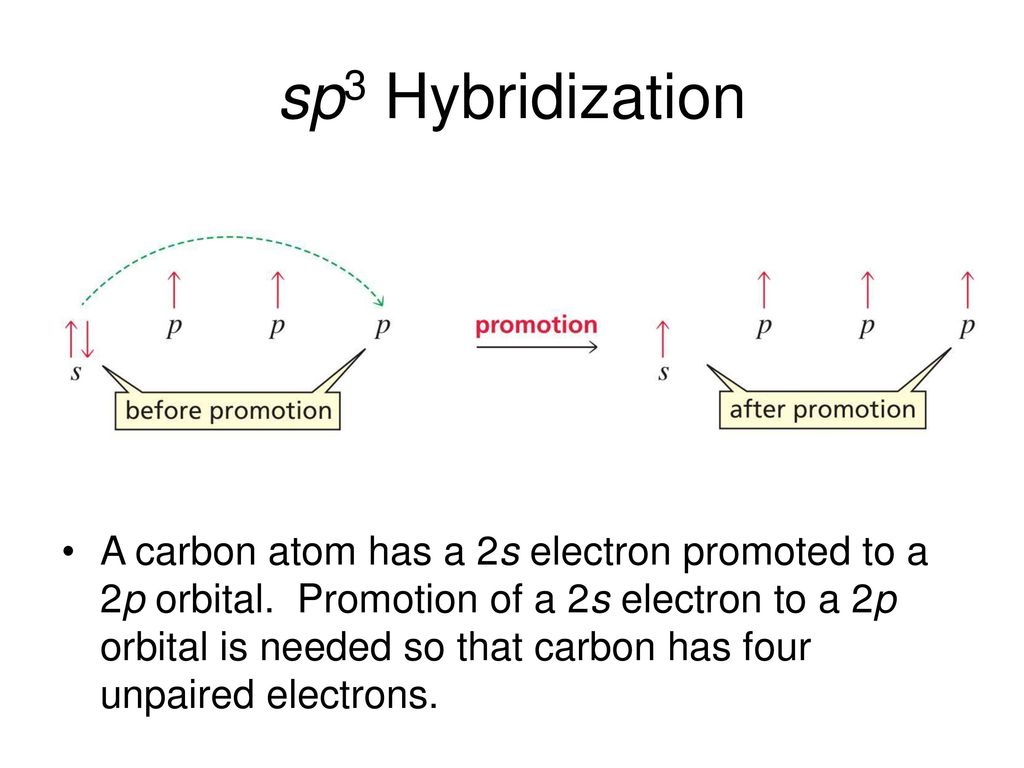
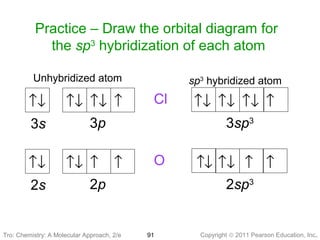
Q. Write orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon before and after sp3 hybridization. Q. Azo dyes are organic dyes that are used for many applications, such as the coloring of fabrics. Many azo dyes are derivatives of the organic... A molecule of methane, CH4, consists of a carbon atom surrounded by four hydrogen atoms at the corners of a tetrahedron. The carbon atom in methane exhibits sp3 hybridization. We illustrate the orbitals and electron distribution in an isolated carbon atom and in the bonded atom in CH4 in Figure 11.
March 2, 2021 - 2. Draw the energy diagram for the orbitals of sp3 hybridzied carbon and nitrogen. Then fill in the correct number of electron. 3. Indicate the hybridization of oxygen in each molecule ... 2. Just like the energy diagram in fig.3. For carbon, each sp3 orbital has 1 electron.
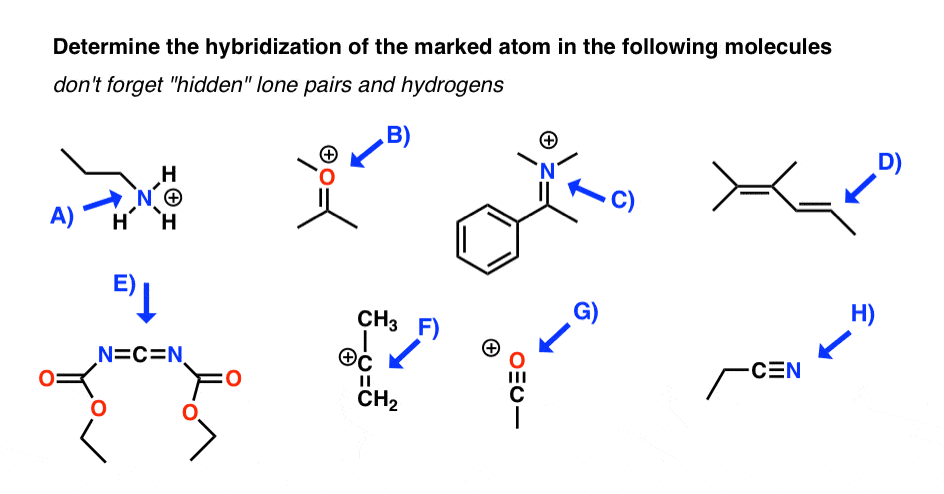
Write the orbital diagram of carbon before sp3 hybridization
Now let's see how hydridisation can account for each of these features, working towards methane then other alkanes: · The 4 sp3 hybrids point towards the corners of a tetrahedron September 10, 2017 - Answer: In CH4, there are 4 sigma bonds, each from the carbon atom to 4 other hydrogen atoms. Carbon has 4 valence electrons- 2 electrons occupying 1 2s and 2 others occupying 2 of the 3 2p orbitals in the second principle quantum shell. The 2s and 2p orbitals thus undergoes hybridisation to form... Answer to: Write the orbital diagram of carbon before sp^3 hybridization. By signing up, you'll get thousands of step-by-step solutions to your...
Write the orbital diagram of carbon before sp3 hybridization. So instead of this being a 2s, ... like for carbon is that this looks like a 2sp3 orbital. This looks like a 2sp3 orbital, that looks like a 2sp3 orbital, that looks like a 2sp3 orbital. They all look like they're kind of in the same orbital. This special type of-- it sounds very fancy. This sp3 hybridized orbital, what ... STEP-5: Assign hybridization and shape of molecule . The hybridization of carbon in methane is sp 3. This molecule is tetrahedral in structure as well as in shape, since there are no lone pairs and the number of σ-bonds is equal to the steric number. The bond angle is 19 o 28'. DETERMINING THE HYBRIDIZATION OF NITROGEN IN AMMONIA, NH 3 STEP-1 ... Write the orbital diagram of carbon before sp3 hybridization. Orbital Hybridization - sp, sp 2, and sp 3 Carbon. Hybridization is used to explain molecular structures and describes the various orbital types which are involved in the bonding between atoms. Answer to write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization. Write the orbital diagram of carbon before sp3 hybridization. Please just explain what the orbital looks like. So no, the atom doesn't have to get excited to 1s2 2s1 2p3 before In the case of sp3 hybridization, say in methane, the carbon s orbital.
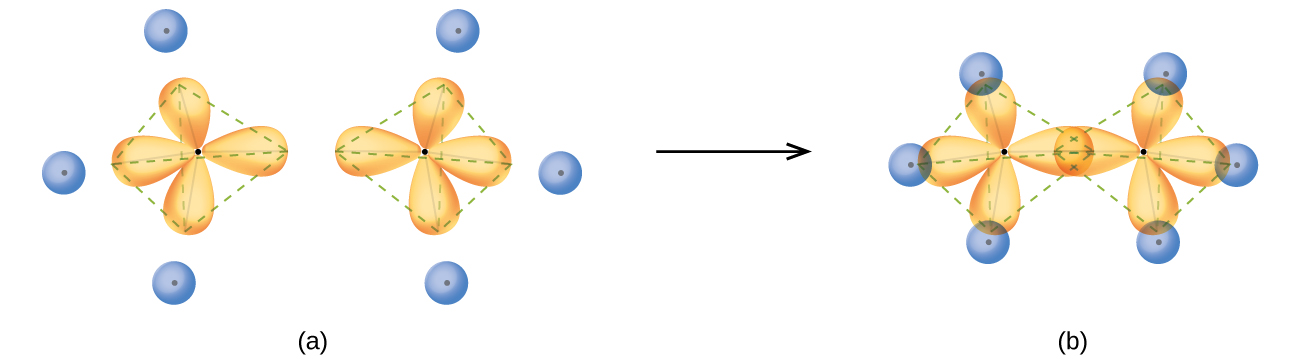
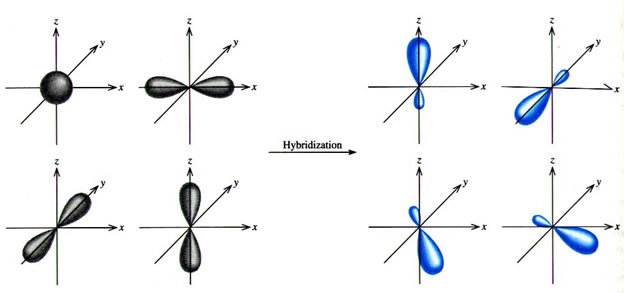
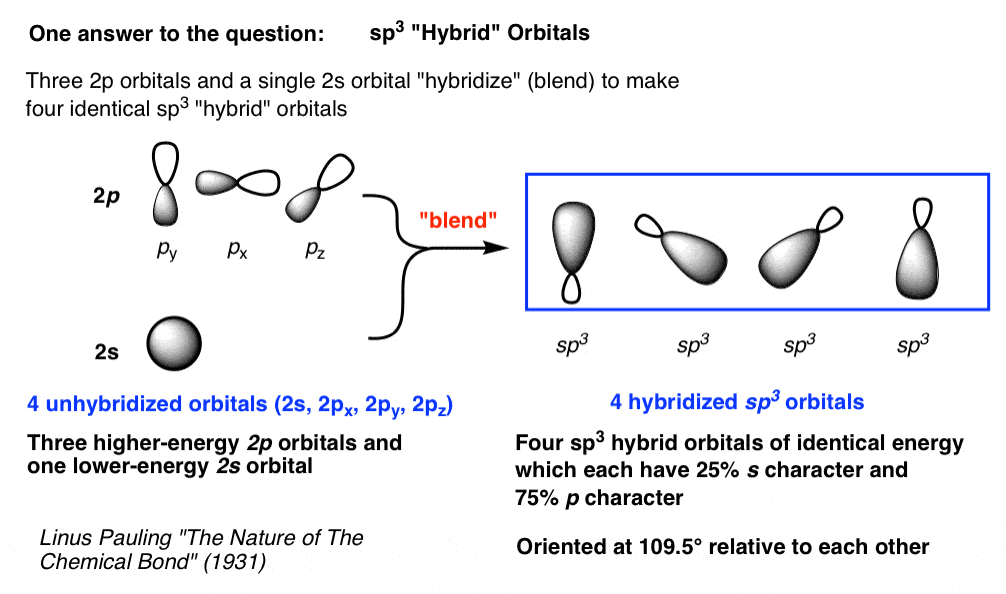
Mar 14, 2016 · Orbital hybridization is essentially a process of mixing orbitals together and spitting out new ones that are all identical in "symmetry" and "composition" to the orbital(s) from the other, incoming atom(s). You can read more about sp^3 hybridization here. The qualitative energies turn out to be the following: with sp^3 hybridized orbitals of 25% s character and 75% p character. SIDENOTE ... An sp3 hybrid orbital is an orbital formed by the linear combination of one s and three p orbitals of comparable energy (such 2s and 2p orbitals) on a same atom. We will now reproduce the sp3 hybridization process for carbon, but instead of taking one s and three p orbitals to make four equivalent sp3 orbitals, this time we'll take only one s and two p orbitals to make three equivalent sp2 orbitals, leaving one p orbital untouched. The process is shown below. 2s 2p X 2p y 2p z Potential energy sp2 ... March 4, 2021 - Therefore, this does not explain how CH4 can exist. To form four bonds the configuration of carbon must have four unpaired electrons. One way CH4 can be explained is, the 2s and the 3 2p orbitals combine to make four, equal energy sp3 hybrid orbitals. That would give us the following configuration:
Methane molecule composed of one carbon atom and four hydrogen atom i.e. CH 4. In methane molecule central atom is carbon. Here carbon atom is Sp 3-hybridized. One s-orbital (2s) and three p-orbital (2px, 2py, 2pz) overlap to produce four Sp 3-hybrid orbitals. These Sp 3 - hybrid orbital are at a angle of 109.5 o from each other. 11. dec. 2019 ... Get the detailed answer: Write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization. The compound is CH4O. The bond angles about the carbon and oxygen are 109.5 degrees because the carbon, 3 hydrogens, and oxygen form a tetrahedral shape with carbon in the center. The hybridization of the carbon and oxygen atoms are both sp3 because they each have 4 regions of electron density. The molecule is polar. November 22, 2019 - The drawing below tries to show how a change in hybridization from sp3 to sp2 brings the p-orbital closer to the adjoining p-orbitals of the pi bond, allowing for better orbital overlap. Better orbital overlap allows for stronger pi-bonding between the nitrogen lone pair and the carbonyl p-orbital, ...
sp3 Hybridization When an s orbital and three p orbital mix, four sp3 hybrid orbitals are formed. Note: the number of orbital before and after mixing are always equal. Methane is a classical example in which the atomic orbitals of carbon are sp3 hybridized.
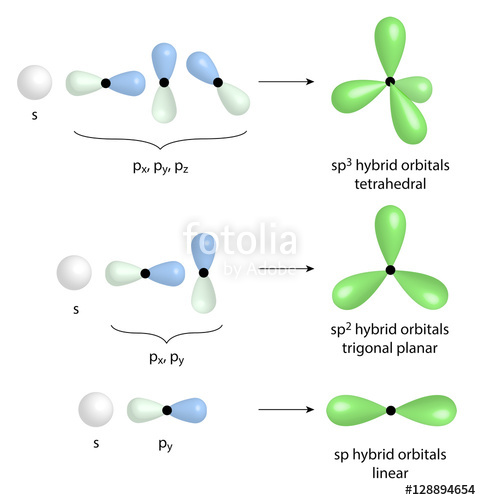
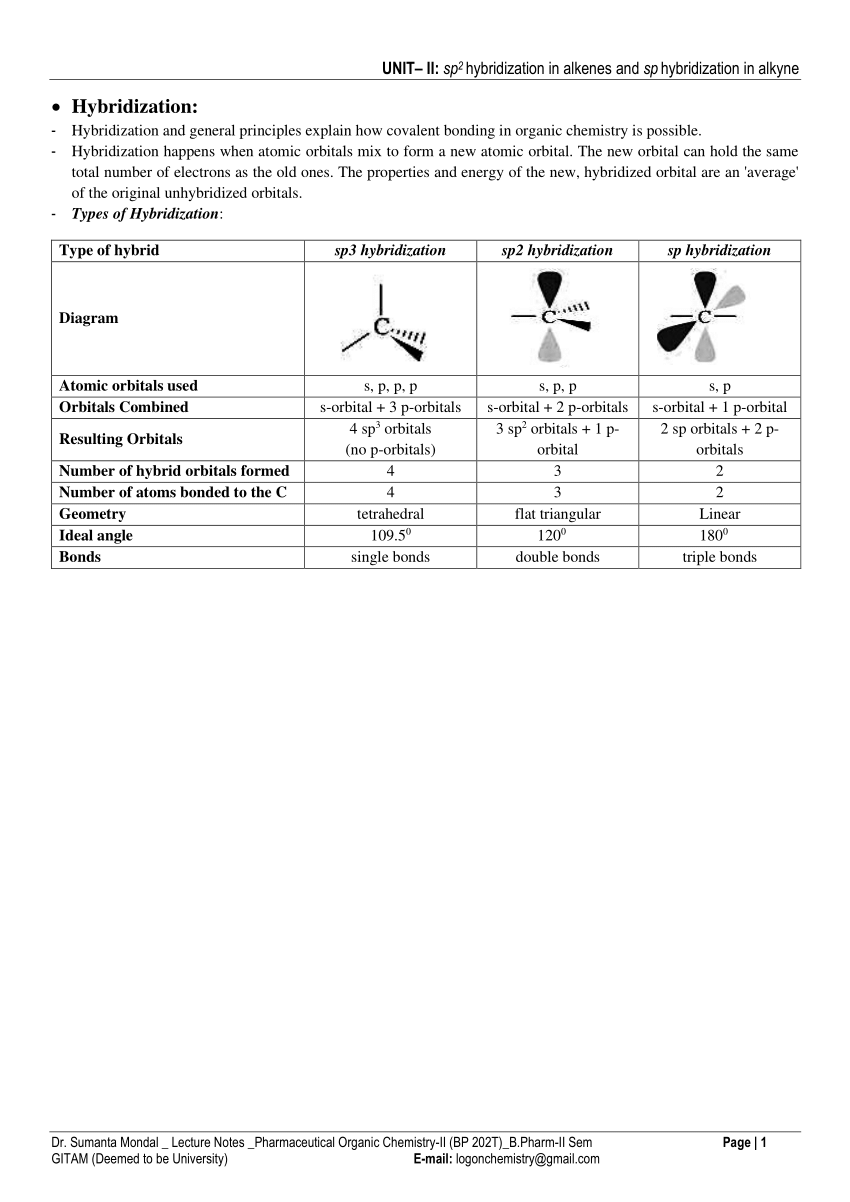
The new orbitals formed are called sp 3 hybrid orbitals. These are directed towards the four corners of a regular tetrahedron and make an angle of 109°28' with one another. The angle between the sp3 hybrid orbitals is 109.28 0; Each sp 3 hybrid orbital has 25% s character and 75% p character. Example of sp 3 hybridization: ethane (C 2 H 6 ...
63: Write a hybridization and bonding scheme for each molecule or ion. Sketch the structure, including overlapping orbitals, and label all bonds including the notation shown in examples 10.6 and 10.7 a. COCl2 (carbon is central atom b. BrF5 c. XeF2 d. I3-...
Consider the electron configuration. Write the orbital diagram of carbon before sp3 hybridization. Question: Consider the electron configuration. Write the orbital diagram of carbon before sp3 hybridization.
The type of hybridization in CO2 is sp hybridization, and each carbon atom forms two sp hybrid orbitals. Out of two hybrid orbitals, one will be used to produce a bond with one oxygen atom, and the other will be used to produce a bond with another oxygen atom. The remaining two p electrons will be used to form a pi (π) bond.
The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. Example: Hybridization of graphite. 3. sp 3 Hybridization. When the carbon atom is bonded to four other atoms the hybridization is said to be sp 3 type. Here 1 s orbital and 3 p orbitals in the same shell of an atom combine to form four new equivalent ...
Write the orbital diagram of carbon before sp3 hybridization. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons.
sp³ hybridization. In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. This type of hybridization is required whenever an atom is surrounded by four groups of electrons. Created by Jay. This is the currently selected item.
19. jul. 2021 ... Write the orbital diagram of carbon before sp3 hybridization. Please just explain what the orbital looks like.
The molecular, sp 3 orbitals are arranged in a tetrahedron, with bond angles of 109.5 o. Each of the 1s orbitals of H will overlap with one of these hybrid orbitals to give the predicted tetrahedral geometry and shape of methane, CH 4. Hybridization also changes the energy levels of the orbitals. The 2s orbital of carbon is lower in energy than the 2p orbitals, since it is more penetrating.
An explanation of the bonding in methane and ethane, including a simple view of hybridisation
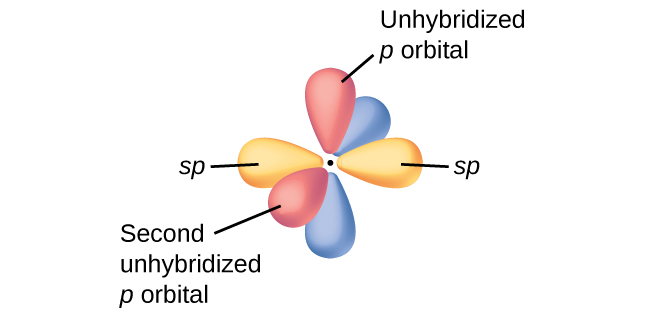
Answer (1 of 3): Hybridisation -C≡C- In a carbon-carbon triple bond ,one is sigma bond and the other two bonds are pi bonds (e g. HC≡CH). * Here carbon undergoes sp hybridization and the two sp hybridized orbitals formed involve in sigma bond formation. * Bonds in H-C≡C-H 1 sigma sp-sp ; 2 s...
Example: Hybridization of CO2. sp2 Hybridization: When carbon atom bonding takes place between 1 s-orbital with two p orbitals then the formation of two single bonds and one double bond between three atoms takes place. Example: Hybridization of graphite. sp3 Hybridization: When the carbon atom is bonded to four other atoms.
Nov 10, 2018 · So no, the atom doesn't have to get excited to 1s2 2s1 2p3 before In the case of sp3 hybridization, say in methane, the carbon s orbital. Orbital hybridization is essentially a process of mixing orbitals together and spitting out new ones that are all identical in "symmetry" and. The atomic number of carbon is 6, which is also the number of The orbital diagram shows how the electrons are arranged within each sublevel. rule, each orbital must contain one electron each with the same spin, before.
For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures which are arranged in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful ...
Q. Write orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon before and after sp3 hybridization. Q. Select the correct hybridization for the central atom based on the electron geometry OF2. Q. The structure of caffeine, present in coffee and many soft drinks, is shown here.
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (6 ratings) Transcribed image text: Write the orbital diagram of carbon before sp hybridization. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons.
Write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization.
Explain the process of hybridization as it applies to the formation of sp3 hybridized atoms. ... The bonds in a methane (CH4) molecule are formed by four separate but equivalent orbitals; a single 2s and three 2p orbitals of the carbon hybridize into four sp3 orbitals.
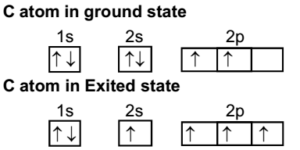
Carbon (atomic number Z=6) in an unbonded state (ground state) has an electronic configuration of 1s2 2s2 2px1 2py1. The electrons in the 1s atomic orbital are ...
Professor Patricia Shapley, University of Illinois, 2012
After sp3 hybridization, the carbon atom has: a. a total of four unpaired electrons ... c. 1 σ bond forms between a hybrid sp orbital on C and an s orbital on H; 1 σ bond forms between a hybrid sp orbital on C and a hybrid sp orbital on N ... Molecular Orbital Diagram-Shows the atomic orbitals of the atoms, the molecular orbitals of the ...
sp hybridization. In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each consisting of 50% s character and 50% p character. This type of hybridization is required whenever an atom is surrounded by two groups of electrons. Created by Jay. This is the currently selected item.
orbital must become unpaired before they can bond. ... The bonds between the sp3 orbitals of hybridized carbon and the s orbitals.
Solved write orbital diagrams to represent the electron | Chegg.com. Science. Chemistry. Chemistry questions and answers. write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization.
August 22, 2008 - Essentially, hybridisation is the ... form new orbitals – which can be used to describe bonding in molecules. Most importantly we have sp3, sp2 and sp hybridisation. ... The best way I can describe sp3 hybridisation is in Methane (also the most basic choice!). This is simplified for expression. Remember that Carbon has 6 ...
27. okt. 2017 ... Write orbital diagrams (boxes with arrows in them) to represent the electron configurations of carbon before and after sp hybridization.
The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. If the atom is neutral, it will have the same number of negatively charged electrons. Its electron configuration is "1s"^2"2s"^2"2p"^2". The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with ...
Write orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon before and after sp3 hybridization. FREE Expert Solution. Before hybridization, we have: 100% (117 ratings) Problem Details.
Step 1. 1 of 2. Let us draw orbital diagram to represent the electron configuration of carbon before and after s p 3 sp^3 s p 3 hybridization. The valence electron configuration: 2 s 2, 2 p 2 2s^2, 2p^2 2 s 2, 2 p 2. When we assign electrons to the orbitals of the same energy, we first fill the orbitals with one electron each with same spin ...
Sp 3 Hybridization. In order to understand why an orbital will engage in hybridization, we need to first look at the electron configuration. 1s 2 2s 2 2p 2 or, written another way, [He]2s 2 2p 2. The s orbital is spherical and can only hold two electrons. In contrast, we have three p orbitals which are lobed and are.
Answer to: Write the orbital diagram of carbon before sp^3 hybridization. By signing up, you'll get thousands of step-by-step solutions to your...
September 10, 2017 - Answer: In CH4, there are 4 sigma bonds, each from the carbon atom to 4 other hydrogen atoms. Carbon has 4 valence electrons- 2 electrons occupying 1 2s and 2 others occupying 2 of the 3 2p orbitals in the second principle quantum shell. The 2s and 2p orbitals thus undergoes hybridisation to form...
Now let's see how hydridisation can account for each of these features, working towards methane then other alkanes: · The 4 sp3 hybrids point towards the corners of a tetrahedron










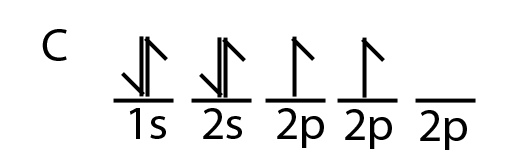






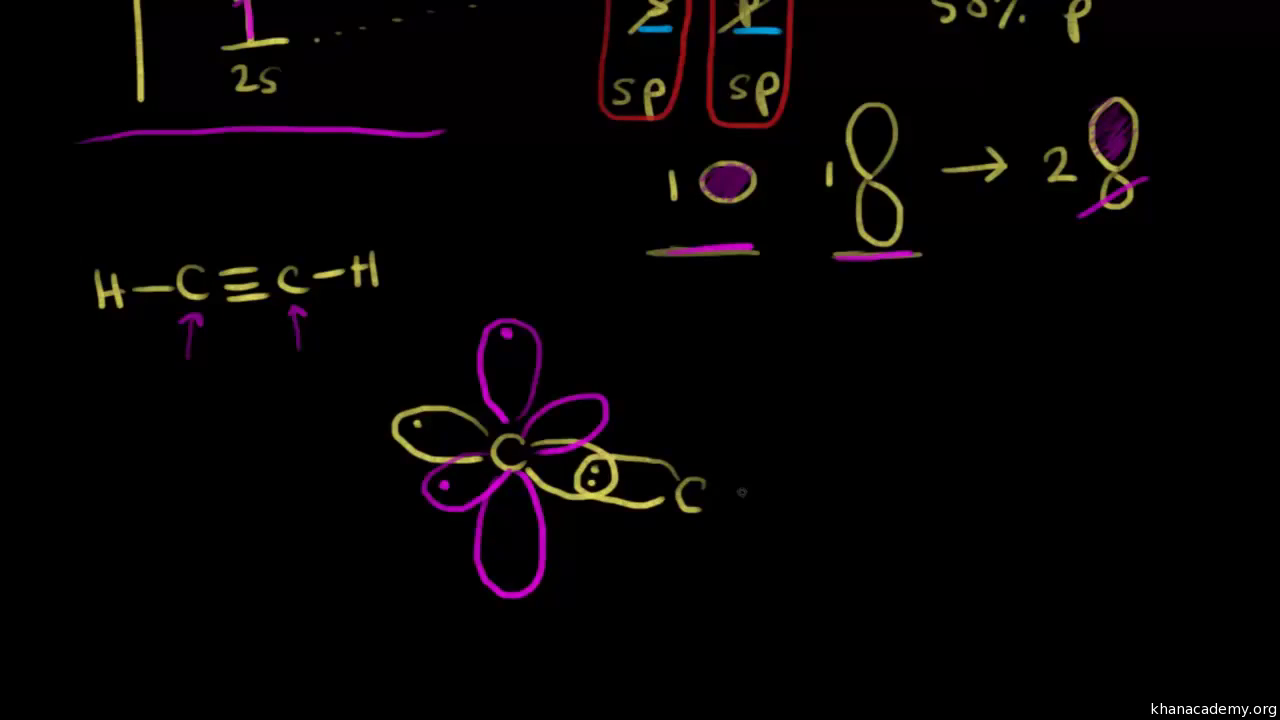

0 Response to "44 write the orbital diagram of carbon before sp3 hybridization"
Post a Comment