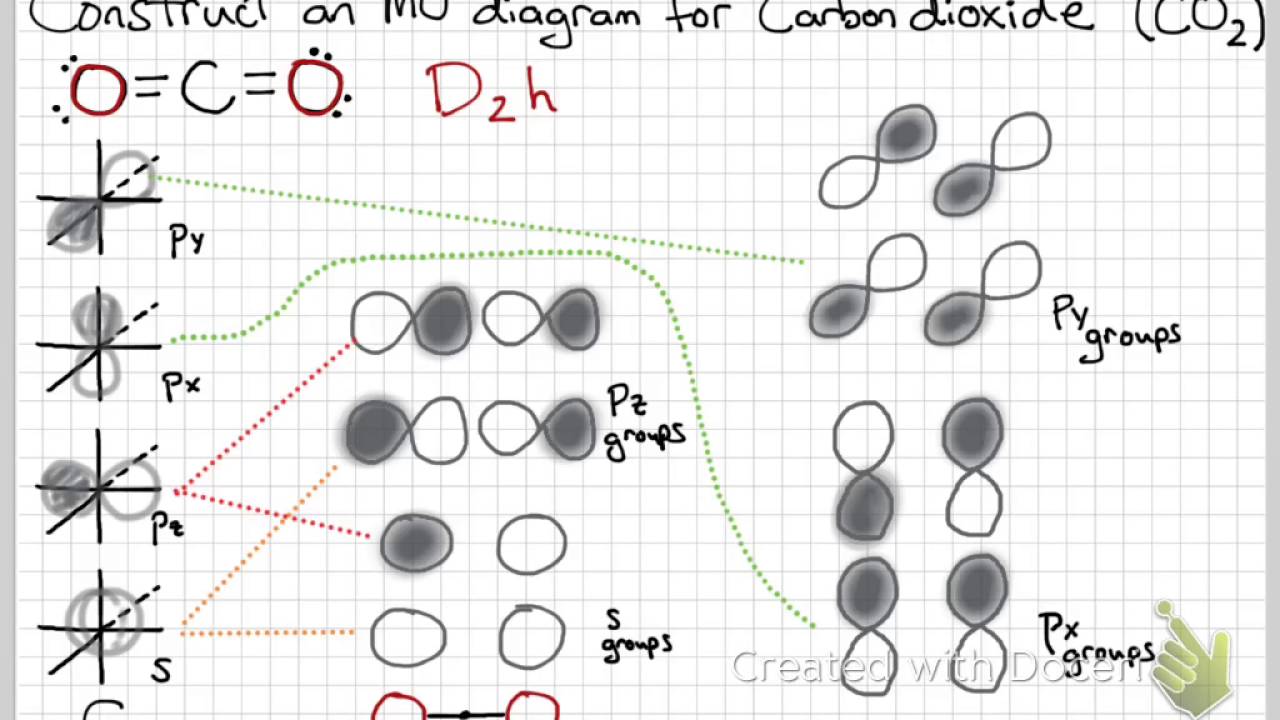
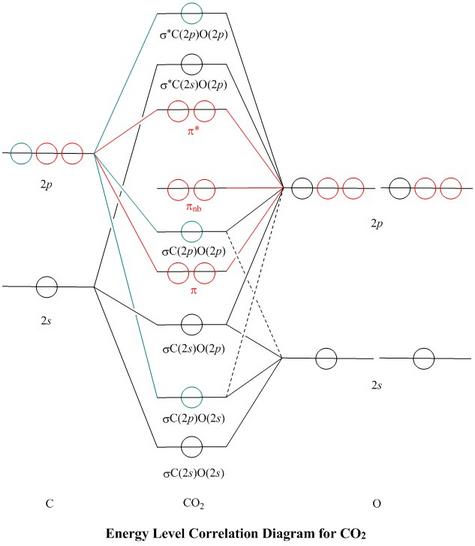
41 co2 orbital overlap diagram
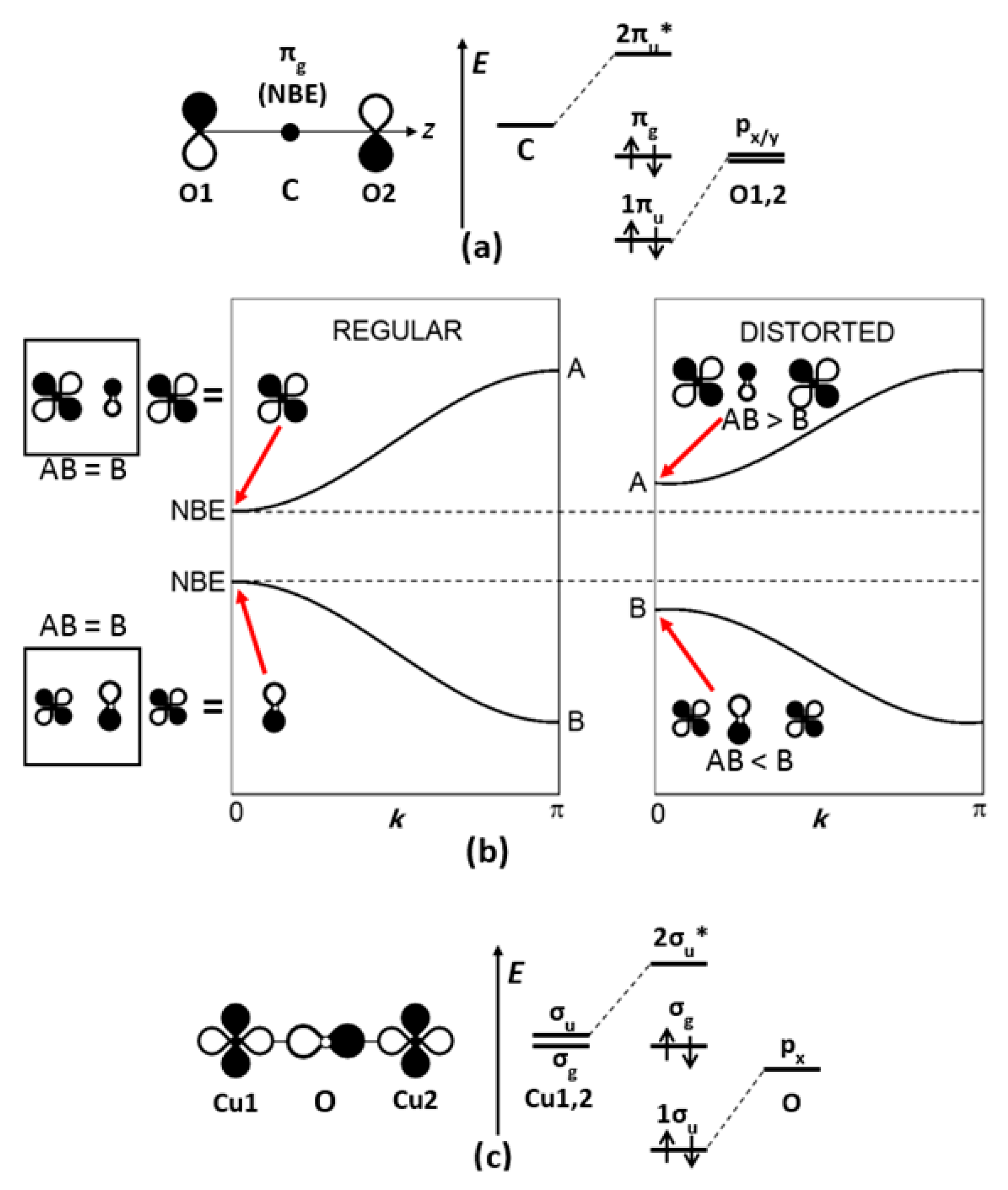
CO2 Lewis Structure (2021 UPDATED) All You Need To Know In a Molecular Orbital Diagram, the 2s orbital of oxygen is nonbonding because of the high energy difference between carbon and oxygen atoms. Based on the rules of the Lewis Structure, all 16 electrons are filled upon bond formation, but the nonbonding orbitals remain vacant, as in the case of CO2. What is linear combination of atomic orbitals for CO2 ... They are shown in its molecular orbital diagram. For example, consider the overlap of the carbon 2s with the 2pz group orbitals of 2 × O. That is labeled 3ag, and is given by Ψ 3ag = 1 √3 [ψ(2s)(C) + ψ(2pz)(O(1)) −ψ(2pz)(O(2))] where ψ is each atomic orbital wave function and Ψ is the molecular orbital wave function.
What is incorrect about the orbital diagram ... What does an orbital diagram tell about a given element? An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally.

Co2 orbital overlap diagram
Draw the orbital diagram for carbon in CO 2 showing how ... Draw the orbital diagram for carbon in CO 2 showing how many carbon atom electrons are in each orbital. Textbook Question. Chapter 8, Problem 31E. ... Ch. 8 - Draw the orbital diagram for carbon in CO2 showing... Ch. 8 - Sketch the distribution of electron density in the... Carbon Monoxide Molecular Orbital Diagram Explanation A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining MO diagrams can explain why some molecules exist and others do not. . combinations such as CO and NO show that the 3σg MO is higher in energy. Mulliken came up with theory known as Molecular Orbital Theory to explain questions like above. What the orbital overlap diagram for SO2 NH3 is a molecule with a nitrogen atom in the middle surrounded by 3 hydrogen atoms bonded covalently. Nitrogen has 5 valence electrons so, it will form sigma bonds (sp3 hybrid orbitals) with each of the hydrogen atom. The remaining lone pair electrons will be situated above the atom. You ca look at this image I've pulled from Google.
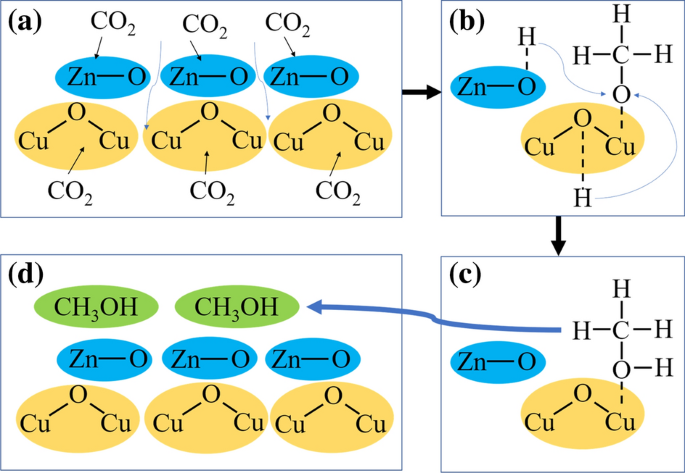
Co2 orbital overlap diagram. how to draw molecular orbital diagram of co - Earn A Lot ... Orbital diagrams use the same basic format but instead of numbers for the electrons they use and arrows as well as giving each orbital its own line to represent the spins of the electrons too. Molecular orbital diagram of N 2 is shown below. Molecular orbitals in Carbon Monoxide. Draw the orbital diagram for the ion Co2. The MO diagram for CO is. Hybridization of CO2 - Hybridization of C, O in Carbon Dioxide Now, these sp hybridized orbitals of the carbon atom overlap with two p orbitals of the oxygen atoms to form 2 sigma bonds. As for the two remaining p electrons they will be used to form a pi bond. In carbon dioxide molecule, oxygen also hybridizes its orbitals to form three sp 2 hybrid orbitals. › exams › bitsatBITSAT 2022 Exam - Dates, Admission Process, Eligibility ... Mar 08, 2022 · Covalent bond: Valence bond theory-orbital overlap, directionality of bonds and hybridization (s, p, and d orbitals only), resonance, molecular orbital theory-methodology, orbital energy level diagram, bond order: Covalent bond: Magnetic properties for homonuclear diatomic species (qualitative idea only) Dipole moments, hydrogen bond Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
PDF Coordination Chemistry II: Jahn-Teller, Square Planar ... Complexes, Orbital Overlap Method, and Electron Counting Chapter 10 and Section 13.3 Monday, November 30, 2015. ... Angular Overlap Method ... σ-Only ML6Octahedral MO Diagram 1eg 2eg 1a1g 2a1g 1t1u 2t1u t2g A1g Eg T1u (n+1)s (n+1)p nd COCl2 Lewis Structure, Molecular Geometry, Hybridization ... COCl2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. COCl2 is a chemical compound, known by the name 'phosgene'. Phosgene is a colorless gaseous compound known as carbonyl chloride and has a molecular weight of 98.92 gram/mol. It is non-flammable in nature and bears a suffocating odor. SCl6, ICl2-, ICl4+, CO2, C2H2 & C2H4 : ORBITAL OVERLAPPING ... Lets learn how to draw the orbital Overlapping Diagram of these molecules:00:01 SCl604:08 ICl2-07:27 ICl4+10:36 CO214:40 C2H221:29 C2H4 Molecular orbital theory(mot) of SF6/CO2/I3-/B2H6 CO2 MOs MO Diagram for CO2 C 2p C 2s bonding MOs antibonding MOs C AOs O LCAOs 1 3 u 3 g 2 u 1 g 1 u 2 u 2 g 1 u 1 g 15. Molecular orbital theory for SF6 molecule- • Electronic configuration of sulphur: • Electronic configuration of Fluorine: • Total number of valence electron: • Hybridization: • Structure of SF6-
team-elektrodynamo.de › JloPWhich Molecule Will Have The Strongest Bond F2 Cl2 O2 N2 As bond order of nitrogen is more than that of oxygen, nitrogen is more stable than oxygen. Although H atoms and H2 molecules are abundant in interstellar space, they are difficult to generate, concentrate and purify on Earth. Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory. › 1814485 › Taiz_and_Zeiger_PlantAcademia.edu Academia.edu pubs.acs.org › doi › 10Polycyclic Aromatic Hydrocarbons (PAHs) in Interstellar Ices ... Feb 21, 2022 · For example, in the PyA complex, the orbital overlap between the LP of ammonia (HOMO A) and the π-orbital of pyrene (HOMO – 2 Py) is larger (S = 0.0266) than the overlap between the LP of water (HOMO W) and the π-orbital of pyrene (HOMO – 2 Py) in the PyW complex, which is only 0.0175 (see Table S2). PDF 2266 Walsh The Electronic Orbitals, Shapes, 2268 Walsh : The Electronic Orbitals, Shapes, nnd molecule. The first of these (b,") is built from a fi atomic orbital on each of the three atoms overlapping in-phase. It is At-tB and (weakly) Bt+B bonding, and becomes one of the lower xu orbitals in the linear molecule.The A atomic orbital concerned in it is pure fi in both bent and linear molecules.
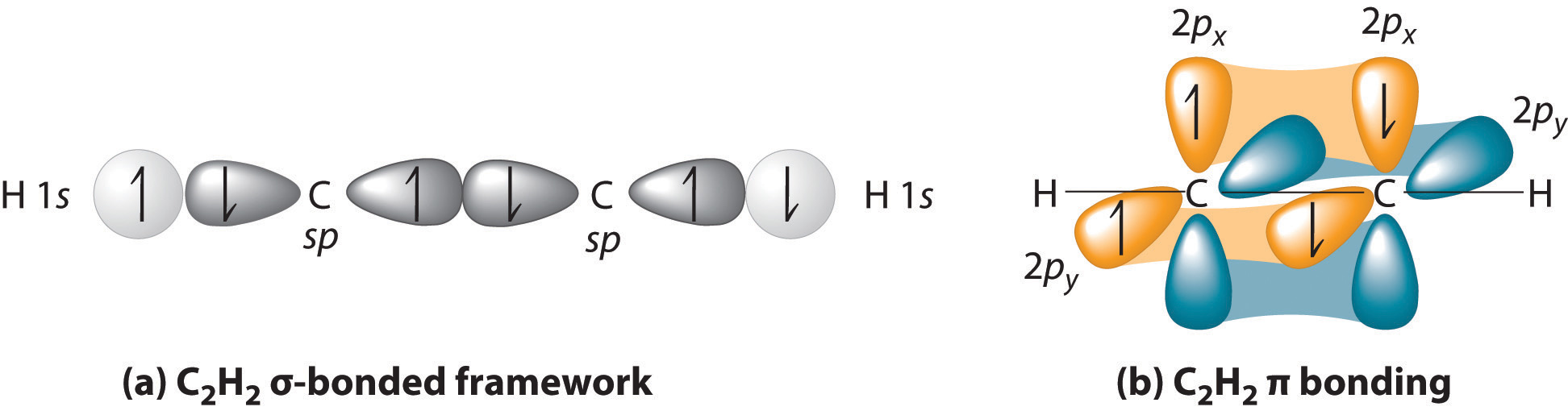
7.7 Molecular Orbital Theory - Chemistry Fundamentals The side-by-side overlap of two p orbitals gives rise to a pi ([latex]\pi[/latex]) bonding molecular orbital and a [latex]\pi[/latex]* antibonding molecular orbital, as shown in Figure 7.7.6. In valence bond theory, we describe π bonds as containing a nodal plane containing the internuclear axis and perpendicular to the lobes of the p-[latex ...
PDF CHAPTER 14 COVALENT BONDING: ORBITALS - UC Santa Barbara from head-to-head overlap of an sp2 orbital from carbon with an sp2 hybrid orbital from oxygen. The π bond is formed from parallel overlap of the unhybridized p atomic orbitals on each atom of C and O. 17. See Exercises 13.51, 13.52, and 13.54 for the Lewis structures. To predict the hybridization,
How To Draw Molecular Orbital Diagram Of Co - Drawing ... Co molecule has 10 valence electrons,four from carbon atom (2s²2p²) and six from oxygen atom (2s²2p⁴).according to molecular orbital diagram, molecular orbital configuration is given as σ2s² σ*2s² πx² πy² σz² π*x⁰ πy⁰ σ*z⁰ Label orbitals sigma, sigma*, pi or pi*.
PDF ORBITALS and MOLECULAR REPRESENTATION In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ...
Co2+ Orbital Diagram The angular overlap diagrams for the molecular orbitals with high d orbital .. For Co2+: High-spin octahedral d7 has LFSE = -∆o. Tetrahedral d7 has. As it is sometimes explained, the statement that 4 s orbital is lower in energy than 3 d But while you fill 3 d orbital with electrons it becomes lower and lower in. Part B.
pubs.acs.org › toc › inocajInorganic Chemistry | Ahead of Print - American Chemical Society Articles ASAP (as soon as publishable) are posted online and available to view immediately after technical editing, formatting for publication, and author proofing.
7.5 Hybrid Atomic Orbitals - Chemistry Fundamentals Explain the concept of atomic orbital hybridization. Determine the hybrid orbitals associated with various molecular geometries. Figure 7.5.1. The hypothetical overlap of two of the 2 p orbitals on an oxygen atom (red) with the 1s orbitals of two hydrogen atoms (blue) would produce a bond angle of 90°. This is not consistent with experimental ...
PDF Hybrid Molecular Orbitals - University of Illinois Urbana ... 6. There is one p orbital on boron but there is no adjacent atom with another p orbital. Add it to the molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1.
manoa.hawaii.edu › exploringourfluidearthStructure and Function - Fish | manoa.hawaii.edu ... External Anatomy of Fishes. Anatomy is the study of an organism’s structures.Fishes come in a diverse array of forms, many with special modifications. The shape, size, and structure of body parts permit different fishes to live in different environments or in different parts of the same environment.
› 35725642 › Chemistry_Gilbert(PDF) Chemistry - Gilbert | Tín Phạm - Academia.edu Academia.edu is a platform for academics to share research papers.
CO2 : ORBITAL OVERLAPPING DIAGRAM - YouTube Some corrections from previous video.. Sorry for the unintentional mistakes i made. So here is the correct one ok.
Hybridization of CO2 - Explanation, Properties, Types ... The formation of CO2 consists of two particles: Oxygen and Carbon. Carbon is in group 4, whereas oxygen is in group 6. Furthermore, there are 2 Oxygen atoms. Therefore, CO2= 4 + 6 (2) = 16. So, the total valence electrons are 16. Carbon is the least electronegative, which means it stays at the centre. So, place the Carbon in the middle and then ...
Molecular orbitals in Carbon Monoxide - ChemTube3D Interactive 3D chemistry animations of reaction mechanisms and 3D models of chemical structures for students studying University courses and advanced school chemistry ...
BeCl2 Lewis Structure, Molecular Geometry, Hybridization ... One 2s orbital and one 2p orbital of Beryllium atom will fuse and form two sp hybrid orbitals of the equivalent energy. These sp hybrid orbitals of Beryllium atom will overlap with 3p orbitals of chlorine atoms and hence, sigma bond formation takes place between Beryllium and chlorine. The same can be represented by its orbital diagram.
Draw The Orbital Diagram For The Ion Co2+ - schematron.org Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the lowest energy orbitals. Click within an orbital to add electrons. Part C. Draw the orbital diagram for the ion N3−.
13.3. Molecular orbitals for three-carbon systems ... If we just take the π molecular orbital and not any of the s, we get three of them. π 1 is bonding with no nodes, π 2 is nonbonding (In other words, the same energy as a regular p-orbital) with a node, and π 3 is antibonding with 2 nodes (none of the orbitals are interacting). The first two electrons will go into the π 1 molecular orbital, regardless of whether it is a cation, radical, or ...
Orbital Overlap Concept - Overlapping of Atomic Orbitals ... In general, the greater the overlap, stronger is the bond formed between the two atoms. Thus, according to the orbital overlap concept, atoms combine by overlapping their orbital and thus forming a lower energy state where their valence electrons with opposite spin, pair up to form covalent bond. The importance of orbital overlap was emphasized ...
Molecular Orbitals for Carbon Monoxide - Newcastle University The Molecule. CO is a very stable 10-valence-electron molecule, isoelectronic with [CN] - and with N 2, which has a slightly lower bond dissociation energy than CO. The formal bond order of CO is 3, from about one σ- bond and two π- bonds. Its most important property is burning in air to give CO 2 , in the combustion of fossil fuels.
What the orbital overlap diagram for SO2 NH3 is a molecule with a nitrogen atom in the middle surrounded by 3 hydrogen atoms bonded covalently. Nitrogen has 5 valence electrons so, it will form sigma bonds (sp3 hybrid orbitals) with each of the hydrogen atom. The remaining lone pair electrons will be situated above the atom. You ca look at this image I've pulled from Google.
Carbon Monoxide Molecular Orbital Diagram Explanation A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining MO diagrams can explain why some molecules exist and others do not. . combinations such as CO and NO show that the 3σg MO is higher in energy. Mulliken came up with theory known as Molecular Orbital Theory to explain questions like above.
Draw the orbital diagram for carbon in CO 2 showing how ... Draw the orbital diagram for carbon in CO 2 showing how many carbon atom electrons are in each orbital. Textbook Question. Chapter 8, Problem 31E. ... Ch. 8 - Draw the orbital diagram for carbon in CO2 showing... Ch. 8 - Sketch the distribution of electron density in the...

















0 Response to "41 co2 orbital overlap diagram"
Post a Comment