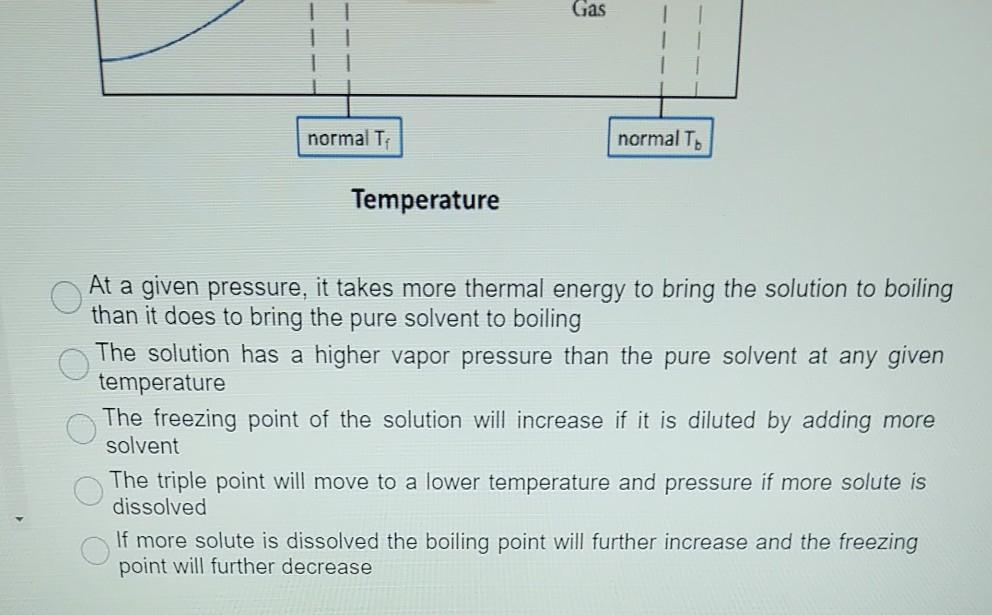
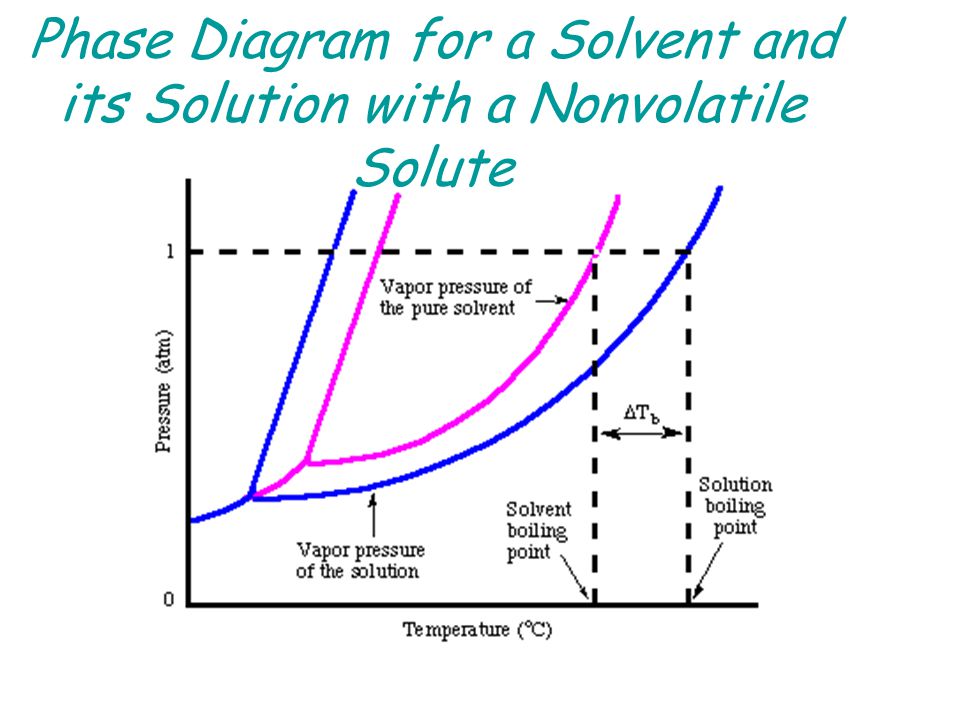
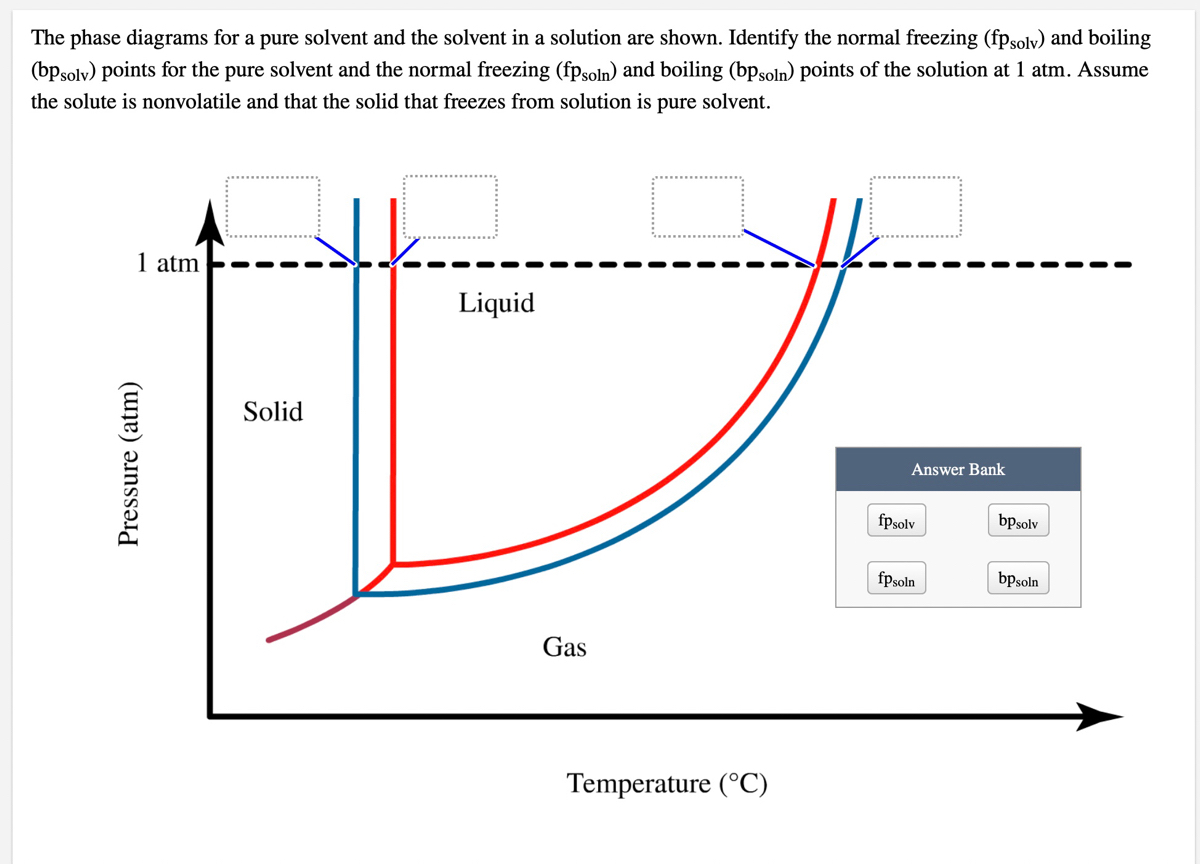
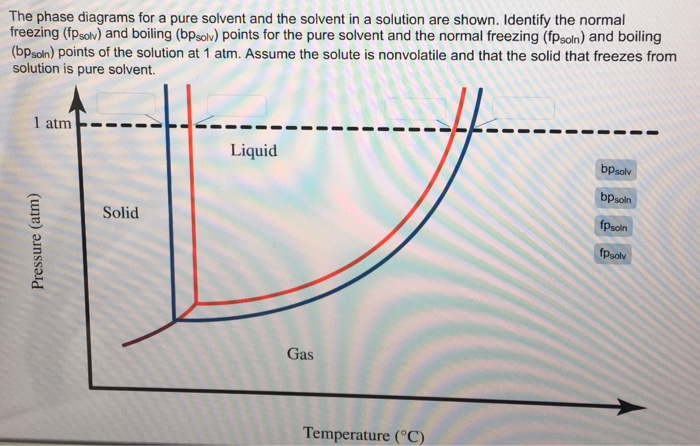
41 label the phase diagram of pure solvent and a solution
PDF Phase Diagrams, Solid Solutions, Phase Transformations Solid solution. Solute Alloys Stainless steels Single-phase alloy Multiple-phase alloy. The phase diagram plots relative concentrations of A and B along the X-axis, and temperature along the Iron-Carbon System. • Pure iron when heated experiences 2 changes in crystal structure before it melts. Phase Diagrams and Solidification (all content) | Pure Substances The phase diagram is a crucial part of metallurgy - it shows the equilibrium states of a mixture, so that given a temperature and composition, it is Consider a mechanical mixture of two phases, A and B. If this is then transformed into a single solution phase with A and B atoms distributed randomly over...
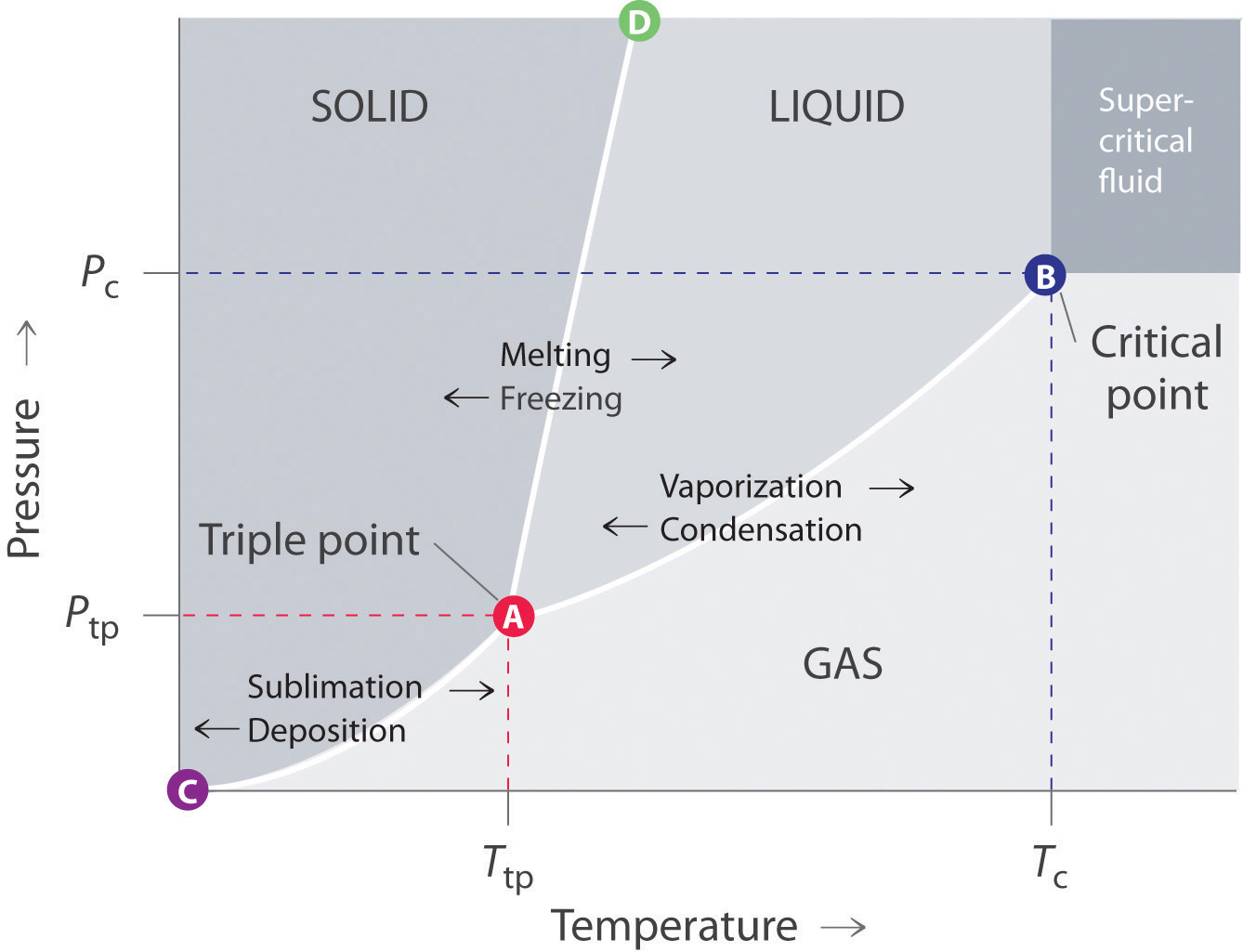
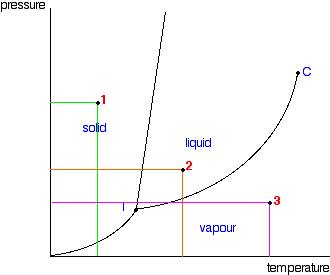
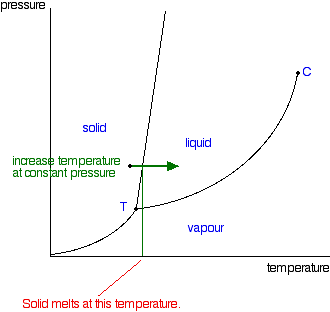
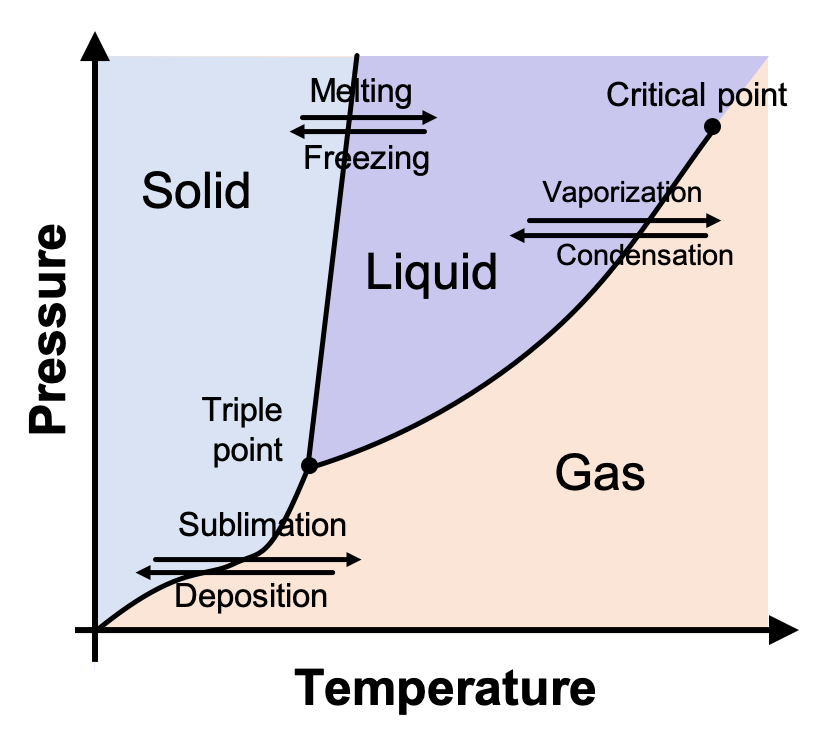
10.4 Phase Diagrams - Chemistry A typical phase diagram for a pure substance is shown in Figure 1. Figure 2. The pressure and temperature axes on this phase diagram of water are not drawn to constant scale in order Solution Using the phase diagram for carbon dioxide provided, we can determine that the state of CO2 at These properties make supercritical fluids extremely useful solvents for a wide range of applications.
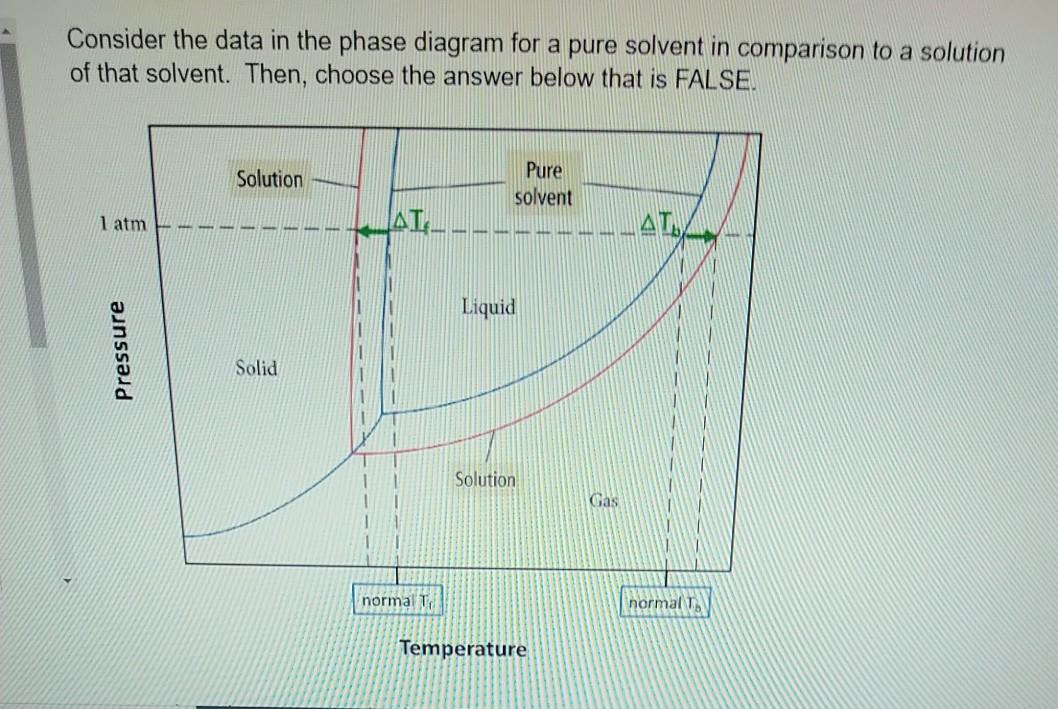
Label the phase diagram of pure solvent and a solution
Isotonic Solution: Definition & Example - Video & Lesson ... 17.09.2021 · An isotonic solution is defined as two solutions of equal concentrations of solutes and water separated by a semipermeable membrane to allow water to move freely in and out of a … Pure Solvent - an overview | ScienceDirect Topics Pure liquids and pure solvents have a unique surface tension value called static or equilibrium A solvent slurry or solvent dope, a solution of a solvent and dissolved thermoplastic pellets, is more Process energy consumption may be reduced below that predicted by the phase-change enthalpy... Label the phase diagram of a pure solvent and a solution. 11 Dec 2019 — Get the detailed answer: Label the phase diagram of a pure solvent and a solution.1 answer · Top answer: Explanation: A phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. ...
Label the phase diagram of pure solvent and a solution. PDF Solutions | Phase diagram Usually, solutes do not dissolve on the solvent vapour (i.e. they are usually non-volatile), neither on the solvent solid phase (they do not is correct for a solution at 25 ºC saturated against pure oxygen at 0 ºC and 100 kPa; if it were against air instead of pure oxygen, the value would be 55·0.21=11.6... Phase diagram of pure solvent and solution. | Download Scientific... Download scientific diagram | Phase diagram of pure solvent and solution. from publication: Design and Evaluation of Multiple Effect Evaporator Systems According to the Steam The evaporation of water from an aqueous solution is widely used in the food, desalination, pulp, and chemical industries. pubchem.ncbi.nlm.nih.gov › compound › 2_3-dimethylbutane2,3-Dimethylbutane | C6H14 - PubChem Vapor-phase 2,3-dimethylbutane is also degraded in the atmosphere by reaction with nitrate radicals(4); the half-life for this reaction in air is estimated to be about 334 days(4,SRC). (1) Bidleman TF; Environ Sci Technol 22: 361-367 (1988) (2) Daubert TE, Danner RP; Physical and Thermodynamic Properties of Pure Chemicals Data Compilation. › science-fair-projectsPaper Chromatography: Is Black Ink Really Black? | Science ... The ink will be spotted onto strips of chromatography paper and put in a beaker containing a solution of alcohol and water. The paper (or more precisely the water that is adsorbed to the paper molecules) is the stationary phase and the alcohol and water solution is the solvent (mobile phase). The solvent will move by capillary action.
Label The Phase Diagram Of Pure Solvent And A Solution Diagram of solvent when discussing solutions the solute is the substance which dissolves while the solvent is the substance into which the 11 solid at ap boiling pointliquid of solution temperature. Show transcribed symbol textual content label the section diagram of pure solvent and an answer. PDF Phase Diagrams Every phase diagram for two or more components must show a liquidus and a solidus, and an A solid solution based on a pure component and extending to certain finite compositions into a binary Another important aspect of interpreting phase diagrams along with phases present is finding the... When a non volatile solute is added to a pure solvent, the molecules to evaporate into the gase phase. in essence, the solute particles obstract the. > When a solution containing non-volatile solute is diluted with water View solution. > The vapour pressure of pure benzene at a certain temperature is 640 mm Hg. > Diagram set. > Memorization tricks. > Cheatsheets. What happens to the solute when a solution freezes? - Chemistry... $\begingroup$ The phase diagram for say pure water and a NaCl/water mixture would be different. The NaCl would lower the freezing point of water. The solute will be found in a small amount of solution that did not freeze, since the solute allows for freezing point depression. So, if you put both...
PDF Solutions and Their Properties. Lecture1 [Режим совместимости] n Any solution consists of a dissolved substance called the solute and the medium in which the solute is uniformly distributed as molecules or ions, called the solvent. n The boiling point of elevation phenomena can be understood by studying phase diagrams of pure solvent and a solution. Cewek Pendiam Ternyata Jago di Ranjang saat Malam Pertama ... 27.02.2022 · Some of the worksheets for this concept are Heating curves work, Heating curve work 1, Practice problems chapter 7 heatingcooling curves, , Chapter 11 heating and cooling curve and phase diagram, Heating and cooling curves, Practice problems chapter 7 heatingcooling curves, Ap ws heating curve calculations key. Lower limit for calculation: -210 C, 0. R = = 15°F … Consider the phase diagram for a pure solvent Recall... | Course Hero What happens to the appearance of the phase diagram when a non-volatile solute is added to the pure solvent? C Osmotic Pressure • consider a pure solvent and a solution of a non-volatile solute separated by semi-permeable membrane that allows solvent but not solute molecules to pass... Phase Diagrams for Pure Substances - Chemistry LibreTexts This page explains how to interpret the phase diagrams for simple pure substances - including a look at the special cases of the phase diagrams of water and carbon dioxide. If you have some ice floating in water, you have a solid phase present and a liquid phase.
Chapter 8 Phase Diagrams A maximum in a phase diagram may occur when favorable interactions between A and B The figure shows the phase diagram of a system in which the liquids become fully miscible before they boil. The system enters the two phase region labeled 'liquid + B'. Pure solid B begins to come out of...
CHAPTER 12, 13, & 14 study guide. Flashcards | Quizlet Label the phase diagram of pure solvent and a solution. ... Which is defined as the maximum amount of solute that will dissolve in a given ... A solution of 3.95 g of anhydrous aluminum chloride (AlCl3) in 56.96 g of water freezes at −3.87°C. Calculate the molar mass of AlCl3 from the formula.
What is an ordered solid solution? - Quora Substitutional solid solution: Solute and solvent atoms distribute over a given crystal lattice sites. of the solvent remains unchanged by addition of the solutes, and when the chemical components remain in a In this case, the pure phase of each element is of the same crystal structure, and the similar...
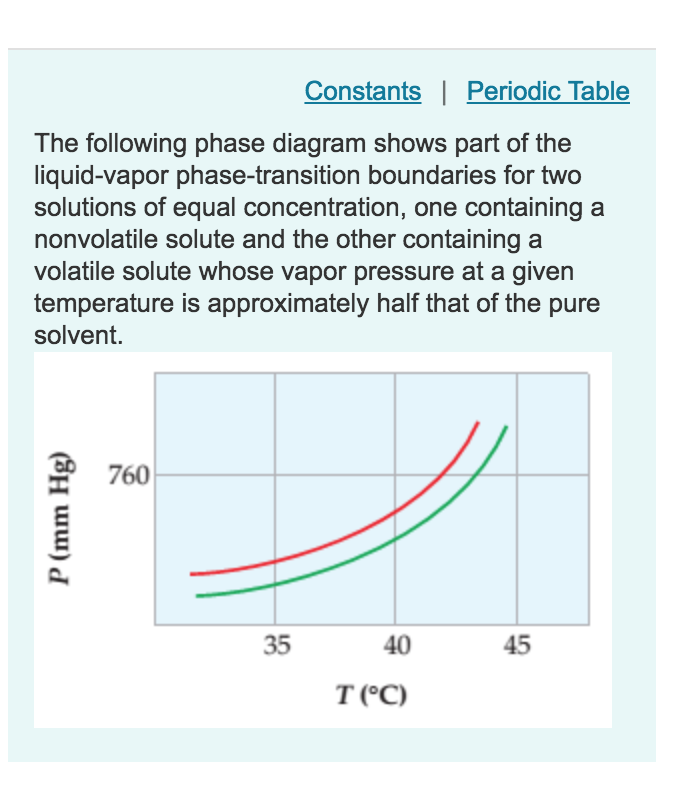
The figure shows two phase diagrams, one for a pure ... 10 Jun 2017 — Now consider the solution made using the pure liquid substance as the solvent, with the phase diagram indicated by the red curves.
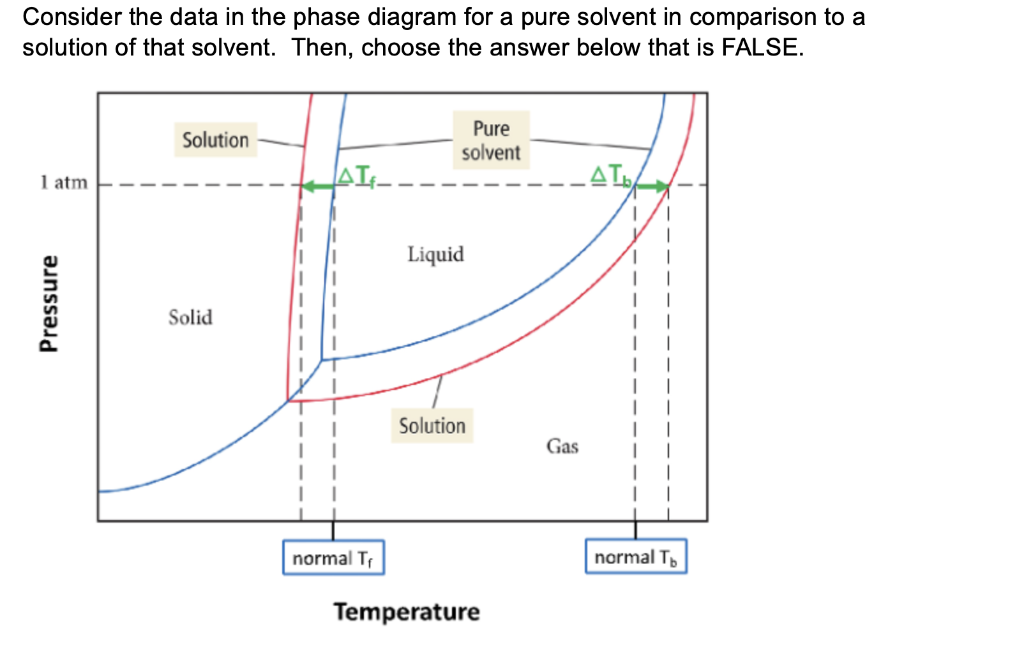
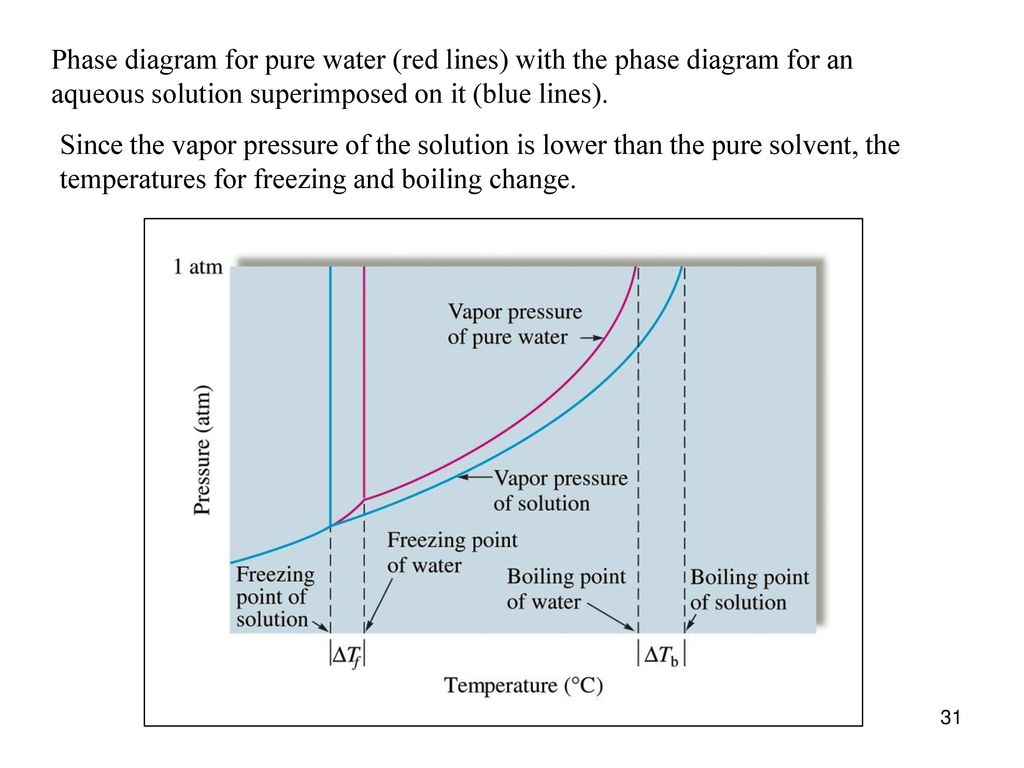
Properties of Solutions Solutes affect some properties of solutions that depend only on the concentration of the dissolved particles. This property is called vapor pressure depressionThe lowering of the vapor pressure of a solution versus the pure solvent. and is depicted in Figure 9.6 "Vapor Pressure Depression".
Calculation of salt precipitation and phase diagrams : Phasediagram Aqueous Salt Solutions. Phase diagrams. The phase diagrams shown on these pages are calculated with the Extended UNIQUAC thermodynamic model. The phase equilibrium line for co-precipitation of potassium chloride and aluminum chloride hexahydrate at 40 °C in this system is shown.
Solvent Extraction Plants: Thiele Diagram & Theoretical Design Aspects Having explained how solvent extractions can be included in a hydrometallurgical flowsheet and outlined some of the large number of interacting variables to be considered, the most basic requirement for solvent extraction design is an understanding of distribution coefficients and equilibrium isotherms.
wou.edu › chemistry › coursesCH103 – Chapter 8: Homeostasis and Cellular Function – Chemistry Solution = Solute + Solvent. Thus, the following equation can be used when calculating percent solutions: Example 1: As an example, a 7.0% v/v solution of ethanol in water, would contain 7 mL of ethanol in a total of 100 mL of solution. How much water is in the solution? In this problem, we know that the: Solution = Solute + Solvent
The Hume-Rothery Rules for Solid Solution - Materials Science... In a substitutional solid solution, the solute atoms will randomly take the place of the solvent atoms. You will end up with two phases: pure oil and a solution of salt water. Hume-Rothery rules only apply in simple cases, and phase diagrams have already been experimentally created for...
Phase Diagrams: The Beginning of Wisdom | SpringerLink Phase diagrams are the perfect road map to understand the conditions for phase formation or transformation in any material system caused by variation of temperature, composition Figure 2 shows the phase diagram of pure iron. This is a unary system, build from a single component.
Solved Label the phase diagram of pure solvent and a | Chegg.com Question: Label the phase diagram of pure solvent and a solution. This problem has been solved! See the answer ...
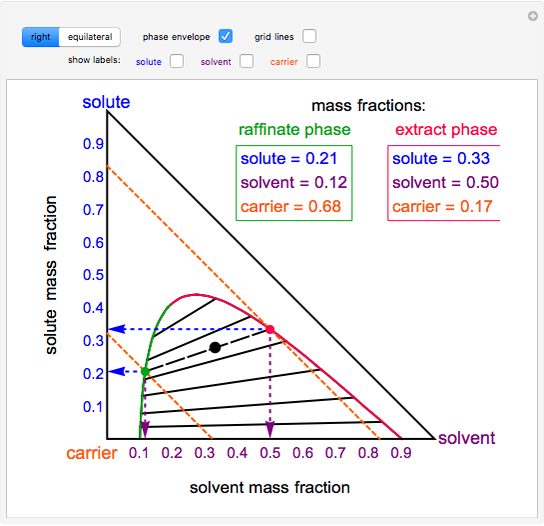
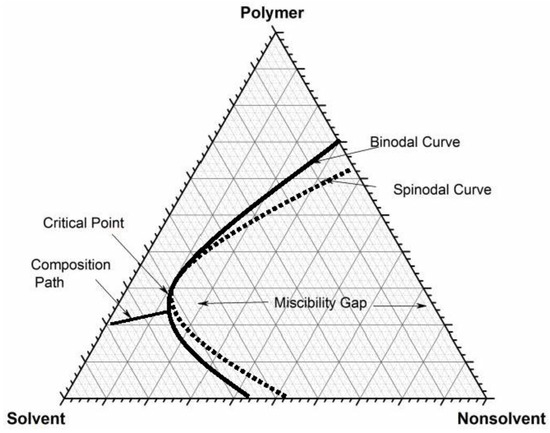
› ternary-phase-diagramTernary Phase Diagram - an overview | ScienceDirect Topics A ternary phase diagram shows possible phases and their equilibrium according to the composition of a mixture of three components at constant temperature and pressure. Figure 4.23 shows a schematic of a ternary phase diagram. Single-phase regions are areas that originate from the vertex of the triangle and that are not enclosed by black curves.
phase diagrams of pure substances This is the phase diagram for a typical pure substance. These diagrams (including this one) are nearly always drawn highly distorted in order to see what is going on more You will have noticed that this liquid-vapour equilibrium curve has a top limit that I have labelled as C in the phase diagram.
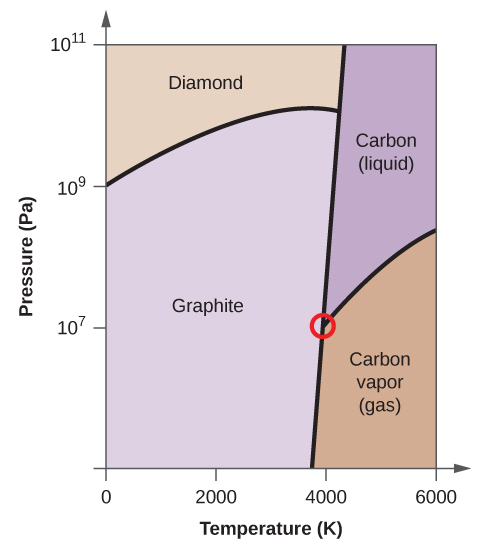
pressbooks-dev.oer.hawaii.edu › phase-diagramsPhase Diagrams – Chemistry Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase.
patapum.to.itFlour Mill Rye [4MH368] Currently unavailable. The developments in grain have made it difficult for some people to digest, but that doesn’t necessarily mean they need to change to a gluten-free diet. It's a coarser, grittier rye than most of the others that I buy online, but it still makes a nice bread. Eureka Rye flour is 100% pure rye with no wheat blended into it.
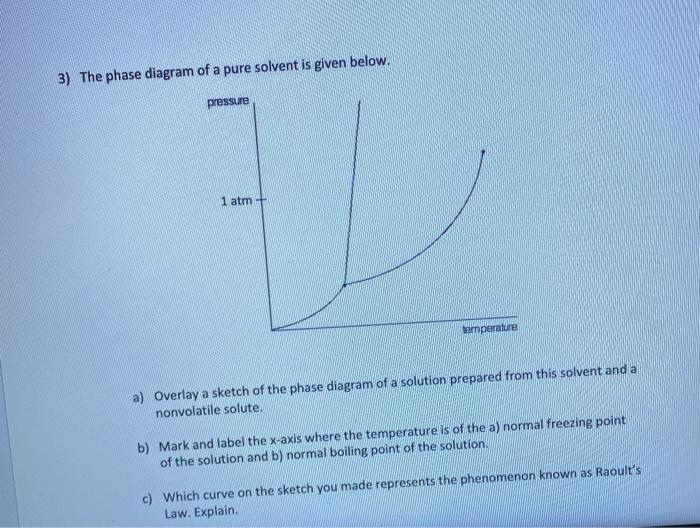
The phase diagram of a pure solvent is given below ... where the temperature is of the a) normal freezing point Mark and label the x-axis of tne solution and b) normal boiling point of the solution. Raoult'$ Which ...
wou.edu › chapter-7-solutionsCH150: Chapter 7 – Solutions – Chemistry Solution = Solute + Solvent. Thus, the following equation can be used when calculating percent solutions: Example 1: As an example, a 7.0% v/v solution of ethanol in water, would contain 7 mL of ethanol in a total of 100 mL of solution. How much water is in the solution? In this problem, we know that the: Solution = Solute + Solvent
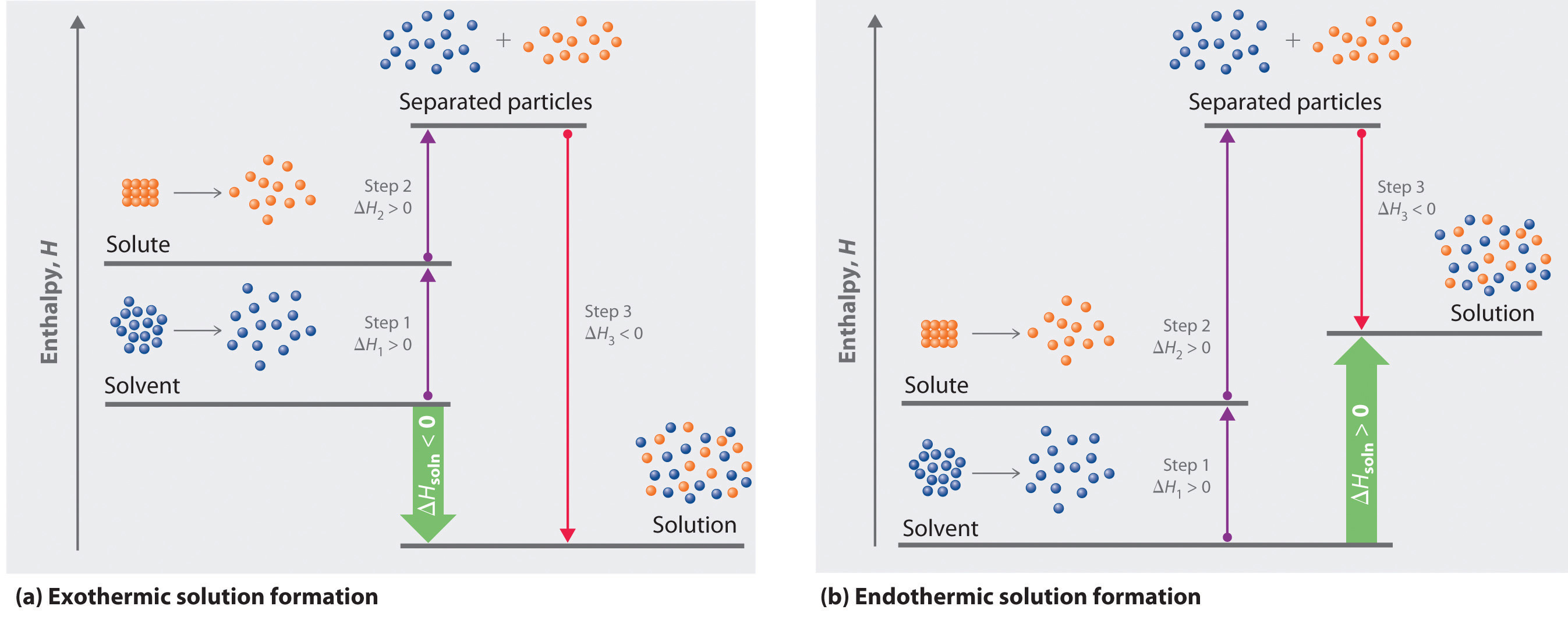
PDF Physical Properties of Solutions When solute and solvent molecules mix to form a solution, there is an increase in randomness or disorder. Much of this order observed in pure state is Figure Phase diagram illustrating the boiling-point elevation and freezing-point depression of aqueous solutions. The dashed curves pertain to the...
Phase diagram - Wikipedia A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically...
2 Component Phase Diagrams | SOLID SOLUTION SYSTEMS ...phase assemblage are labeled. the resulting phase diagram is called a binary eutectic diagram. Figure 1 shows the simplest of two component phase diagrams. The components are A and B, and Because this composition falls between the compositions of pure forsterite and pure enstatite, it must...
Ideal and Real Solutions | PDF | Phase (Matter) | Phase Diagram dissolves until the solution is saturated with the dissolved solute. Osmosis The equilibrium involved in the calculation of osmotic pressure, Π , is between pure solvent A at a pressure pon one side of the semipermeable membrane and A as a component of the mixture on the other side of the membrane...
35 Label The Phase Diagram Of Pure Solvent And A Solution For example the addition of a solute to a pure solvent making a solution. The vaporization curve for the solution lies below the cur...
PDF Solution and Solubility | Binary Phase Diagrams The phase diagram of water is a common example. Water may stay in liquid, solid or gaseous states in different pressure-temperature regions. The simplest type of binary phase diagrams is the isomorphous system, in which the two constituents form a continuous solid solution over the entire...
2.2: Molecular Weight Determination - Chemistry LibreTexts 21.03.2021 · Allow the solution to stir continuously to avoid supercooling. Weigh (15 to 20 grams) of the pure solvent in a test tube and record the measured weight value of the pure solvent. Place a stir bar or wire stirrer in the test tube and close with a rubber stopper that has a hole to encase a mercury thermometer.
Label-Free Optical Analysis of Biomolecules in Solid-State ... 25.02.2022 · Then, label-free optical methods of localized surface plasmon resonance detection and surface-enhanced Raman spectroscopy on solid-state plasmonic nanopores with different configurations will be described in detail to show how they approach and address the discrimination of the 20 amino acids at the single-molecule level. It is worth mentioning that we …
Label the phase diagram of a pure solvent and a solution. 11 Dec 2019 — Get the detailed answer: Label the phase diagram of a pure solvent and a solution.1 answer · Top answer: Explanation: A phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. ...
Pure Solvent - an overview | ScienceDirect Topics Pure liquids and pure solvents have a unique surface tension value called static or equilibrium A solvent slurry or solvent dope, a solution of a solvent and dissolved thermoplastic pellets, is more Process energy consumption may be reduced below that predicted by the phase-change enthalpy...
Isotonic Solution: Definition & Example - Video & Lesson ... 17.09.2021 · An isotonic solution is defined as two solutions of equal concentrations of solutes and water separated by a semipermeable membrane to allow water to move freely in and out of a …
















0 Response to "41 label the phase diagram of pure solvent and a solution"
Post a Comment