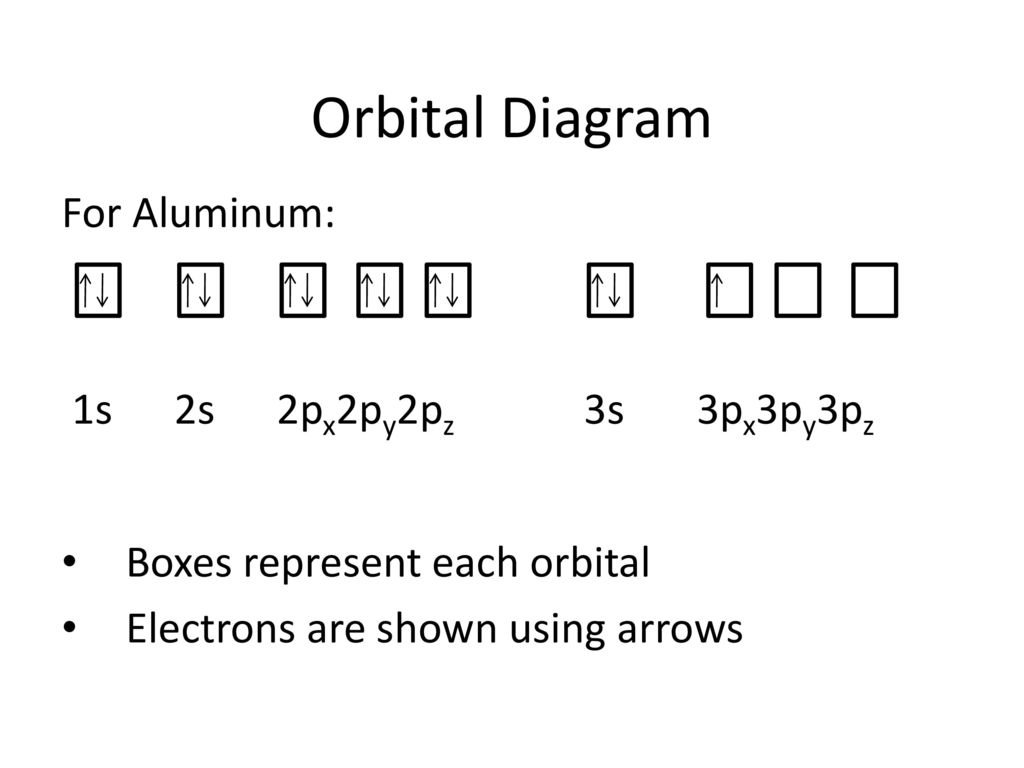
41 orbital diagram of aluminum
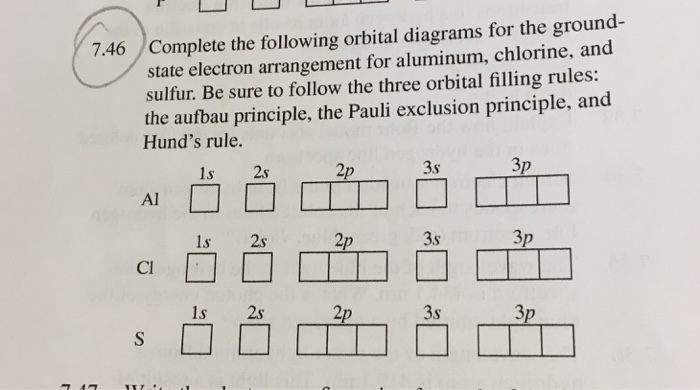
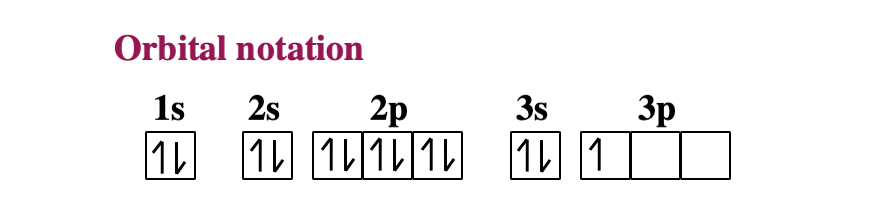
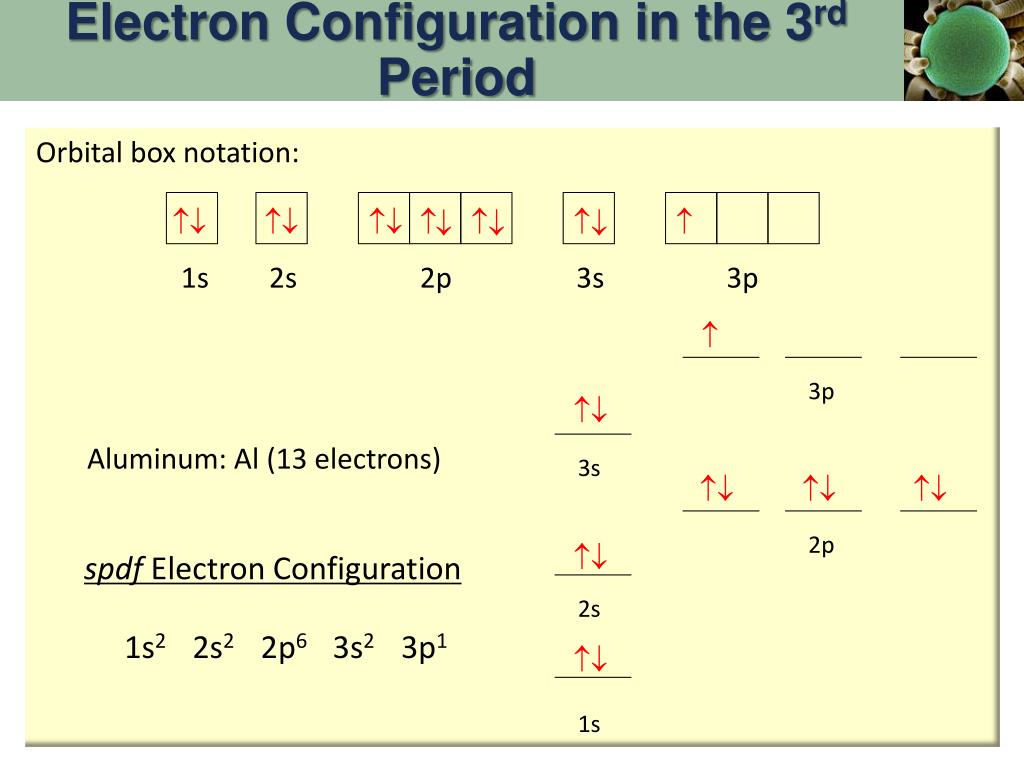
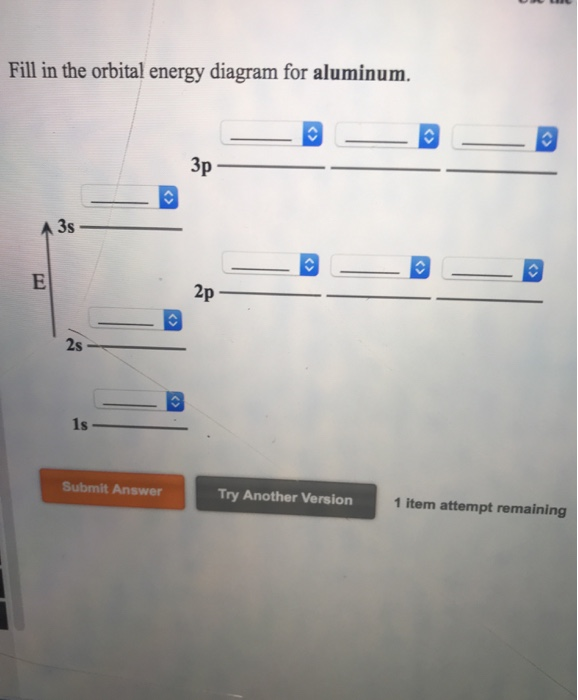
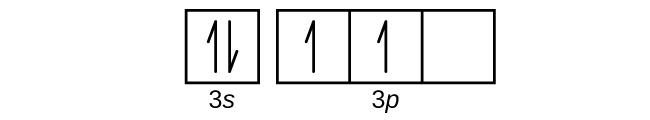
6.4 Electronic Structure of Atoms (Electron Configurations ... The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ). orbital notation for aluminum - jamesandjeffreyantiques.com An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represe nt the electrons in each orbital. Refer to the related link to see an illustration of an orbital diagram for aluminum. 1s2 2s2 2p6 3s2 3p1.
Exceptions to the Octet Rule | Boundless Chemistry Valence electrons can be counted using a Lewis electron dot diagram. In carbon dioxide, for example, each oxygen shares four electrons with the central carbon. These four electrons are counted in both the carbon octet and the oxygen octet because they are shared. Carbon dioxide: A Lewis dot diagram for carbon dioxide. Hydrogen and Lithium. However, many atoms below …
Orbital diagram of aluminum
Aluminum Bohr Model - How to draw Bohr diagram for ... The Bohr model of Aluminum (Al) is drawn with three electron shells, the first shell contains 2 electrons, the second shell contains 8 electrons and the third shell contains 3 electrons. Aluminum is neutral and its atomic number is 13, hence, the number of protons and electrons available for its Bohr diagram is also 13. PDF Shells, Subshells, and Orbitals - The SHAPE of an orbital is defined by the SUBSHELL it is in ... 159 ENERGY DIAGRAM - We can map out electrons around an atom using an energy diagram: E N E R G Y 1s 2s 2p 3s 3p 3d 4s 4p 4d 5s 5p ... Aluminum: Z = 13 Aluminum has THREE valence electrons! (All electrons in the outer shell are valence How to Write the Orbital Diagram for Aluminum (Al) - YouTube To write the orbital diagram for the Aluminum atom (Al) first we need to write the electron configuration for just Al. To do that we need to find the number ...
Orbital diagram of aluminum. What is the orbital diagram of aluminum? - Quora What is the orbital diagram of aluminum? 1 Answer Eric Snyder , studied Chemistry & Physical Chemistry at UCLA (1992) Answered 1 year ago · Author has 143 answers and 32.4K answer views Aluminum (Al) has only atomic orbitals. As a 3rd row element, however, it has a complete Ne electron configuration. what is the orbital diagram of aluminum - Brainly.ph What is the orbital diagram of aluminum - 9348602 2. Is there a change in the form or appearance of the material? 3. What can you say about the temperature when the material was cooled 4. Aluminum - Basics The orbital diagram of aluminum helps show the specific "address" of each electron. Each arrow in the diagram represents a single electron (arrow pointing up if positive spin, down if negative spin). The numbers above the squares of the diagram represent the energy level, and the letters represent sub levels. Each box represents an orbital, also. Aluminum Orbital diagram, Electron configuration, and Valence ... The orbital diagram for Aluminum is drawn with 5 orbitals. The orbitals are 1s, 2s, 2p, 3s, and 3p. The Aluminum orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, the six electrons in 2p orbital, the two electrons in 3s orbital, and the remaining one electron in 3p orbital.
What is the electron dot diagram for aluminum ... What is the electron dot diagram for aluminum? Give the electron dot structure for aluminum. Answer: Aluminum is in group IIIA of the periodic table therefore it has three valence electrons. The symbol for aluminum is Al which will be surrounded by three dots. ... This orbital is equivalent to the innermost electron shell of the Bohr model of ... What is the orbital diagram for aluminum? - Answers An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represent the electrons in ... Chapter 5: Electrons in Atoms Flashcards - Quizlet Write the orbital diagram of aluminum. Orbital: Aluminum - 13. Write the complete electron configuration and the noble-gas notation for aluminum. Configuration: 1s2, 2s2, 2p6, 3s2, 3p1 Noble-Gas Notation: [Ne]3s2,3p1. Write the complete noble-gas notation for iodine. [Kr]5s2, 4d10, 5p5. Give the orbital diagram for aluminum. | Study.com To determine the electron configuration and draw an orbital diagram of aluminum, we follow 3 rules: Aufbau principle: This states we fill starting from lower energy levels and progress to the next ...
Sodium Bohr Model - How to draw Bohr diagram for Sodium(Na ... Aluminum Bohr model; Potassium Bohr model; Calcium Bohr model; Bromine Bohr model; Find Valence electron of Sodium through its Bohr diagram. Valence electrons are found in the outermost shell of an atom and they can take participate in the formation of a chemical bond. These electrons have more energy compare to the inner shell electrons. From the Bohr … Aluminum(Al) electron configuration with orbital diagram Orbital diagram for aluminum(Al) The next three electrons will enter the 2p orbital in the clockwise direction and the next three electrons will enter the 2p orbital in the anti-clockwise direction. The next two electrons will enter the 3s orbital and the remaining one electron will enter the 3p orbital in a clockwise direction. Electron Configuration for Aluminium (Al) In writing the electron configuration for Aluminium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for aluminium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. Aluminum Bohr Diagram A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Jan 18, · Aluminum, Al Bohr Diagram.
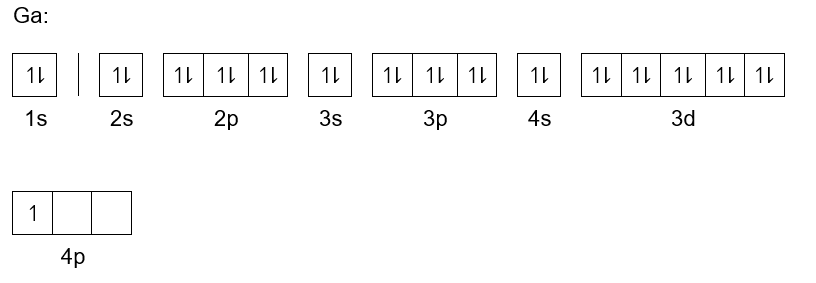
Electron configuration for Aluminum (element 13). Orbital ... Melting point: 660.5 ℃. Density: 2.7 g/cm 3 . Electronic configuration of the Aluminum atom: 1s 2 2s 2 2p 6 3s 2 3p 1. Reduced electronic configuration Al: [Ne] 3s 2 3p 1. Below is the electronic diagram of the Aluminum atom Distribution of electrons over energy levels in the Al atom. 1-st level (K): 2. 2-st level (L): 8.
What is the orbital diagram of aluminum? - Quora Aluminum (Al) has only atomic orbitals. As a 3rd row element, however, it has a complete Ne electron configuration. One way to denote this is to write it as the following: [Ne] 3s (2) 3p (1), The parentheses indicate the number of electrons in each orbital. The full electron shell (including the Ne configuration) is:
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus ...
How to Write the Orbital Diagram for Aluminum (Al) - YouTube To write the orbital diagram for the Aluminum atom (Al) first we need to write the electron configuration for just Al. To do that we need to find the number ...
PDF Shells, Subshells, and Orbitals - The SHAPE of an orbital is defined by the SUBSHELL it is in ... 159 ENERGY DIAGRAM - We can map out electrons around an atom using an energy diagram: E N E R G Y 1s 2s 2p 3s 3p 3d 4s 4p 4d 5s 5p ... Aluminum: Z = 13 Aluminum has THREE valence electrons! (All electrons in the outer shell are valence
Aluminum Bohr Model - How to draw Bohr diagram for ... The Bohr model of Aluminum (Al) is drawn with three electron shells, the first shell contains 2 electrons, the second shell contains 8 electrons and the third shell contains 3 electrons. Aluminum is neutral and its atomic number is 13, hence, the number of protons and electrons available for its Bohr diagram is also 13.





















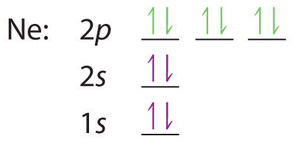





0 Response to "41 orbital diagram of aluminum"
Post a Comment