41 reaction coordinate diagram endothermic vs exothermic
Arrhenius Theory and Reaction Coordinates - Chemistry 302 Typically, we envision reactions proceeding left to right along the reaction coordinate, so often, the activation energy is only noted for the forward reaction. The activation energy on the diagram below shows the barrier to be 102.6 kJ mol -1 . Endothermic Vs. Exothermic - Blogger The reason why the products are lower is beacuse Exo means out, so the reactants lose energy. The reaction Coordinate is just the run time. The Endothermic Graph looks like this: (Ignore the Delta E it's supposed to be Delta H) The reason why the products are higher is because Endo means in, so the reactants gain energy.
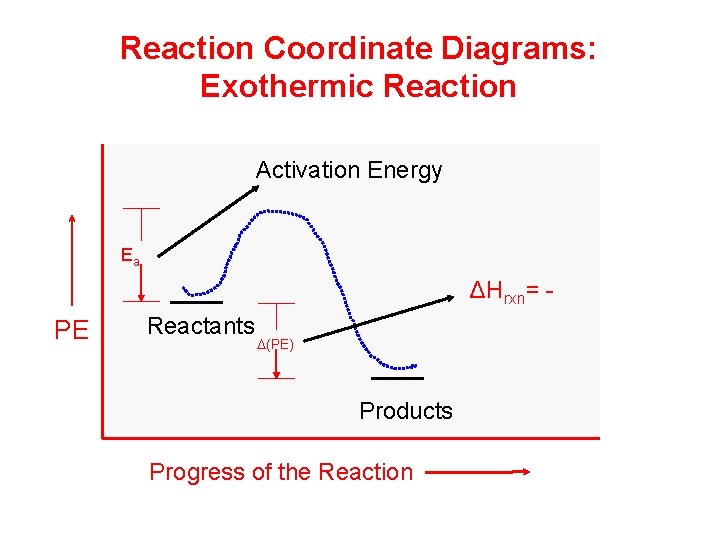
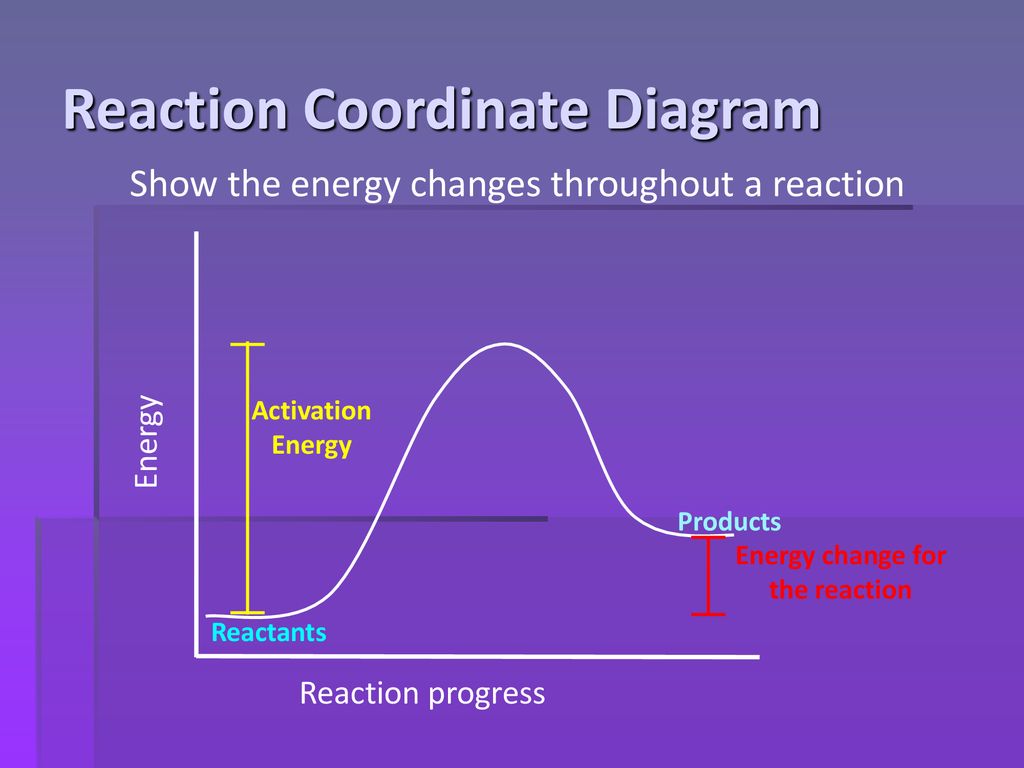
Reaction Coordinate Diagrams - University of Illinois ... The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction(heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram.
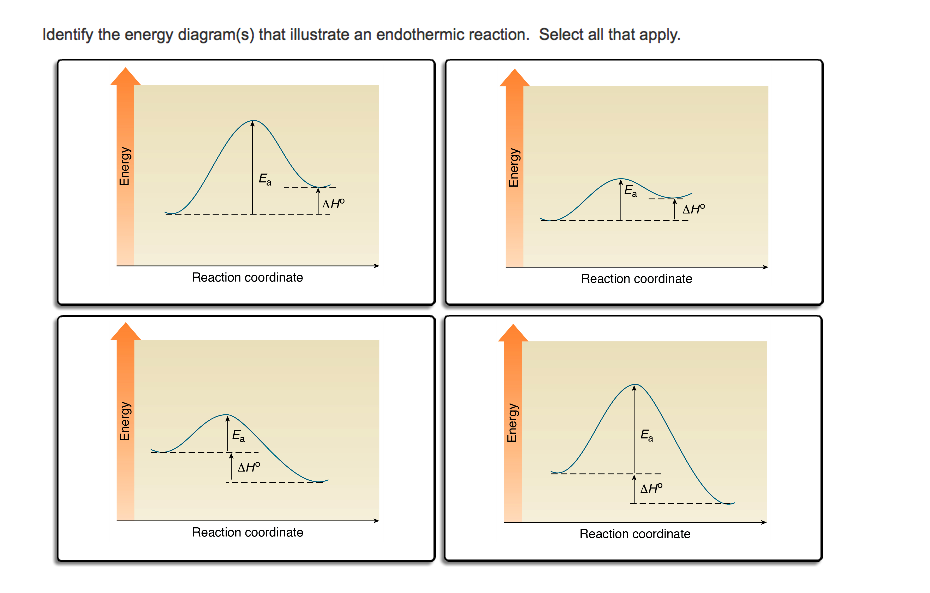
Reaction coordinate diagram endothermic vs exothermic
Stunning Endothermic Exothermic Reaction Equation Notes Of ... The exothermic reaction is the opposite of an endothermic reaction. Fusion of snow on a warm windshield especially for heavy snow. A good example of an endothermic reaction is photosynthesis. The chemical equation can be written as follows. Conversely an exothermic reaction is one in which energy is released from the system into the surroundings. Reaction profiles - Exothermic and endothermic reactions ... Reaction profiles An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them.... PDF NAME Kinetics Potential Energy Diagrams - ISD 622 The potential energy diagrams for the exothermic reaction ... difference between the diagrams for the exothermic vs. endothermic reactions? _____ Explain _____ ... Reaction Coordinate X + Y + energy XY (continued) 7. On the diagram below, draw a dotted line to show the pathway of a catalyzed reaction. Add the
Reaction coordinate diagram endothermic vs exothermic. Fountain - Custom Essay Writing Service - 24/7 ... 100% money-back guarantee. With our money back guarantee, our customers have the right to request and get a refund at any stage of their order in case something goes wrong. Endothermic Reaction Coordinate Diagram A reaction is endothermic when the energy of the products is greater than the energy of the reactants. The is for an exothermic reaction. Below is a reaction coordinate diagram for an endothermic reaction. A reaction coordinate diagram shows the energy changes that take place in each of the steps of the mechanism. Endothermic Vs Exothermic - CHEMISTRY COMMUNITY Re: Endothermic Vs Exothermic. When a system absorbs heat from its surrounding, we say that the reaction is endothermic and has a positive delta H. This is because the enthalpy of products is greater than the enthalpy of reactants. Since there is more energy on the product's side of the reaction, the change (symbolized as delta H) is positive. Endothermic vs. exothermic reactions (article) | Khan Academy Energy diagrams for endothermic and exothermic reactions In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, the products are less stable than the reactants.
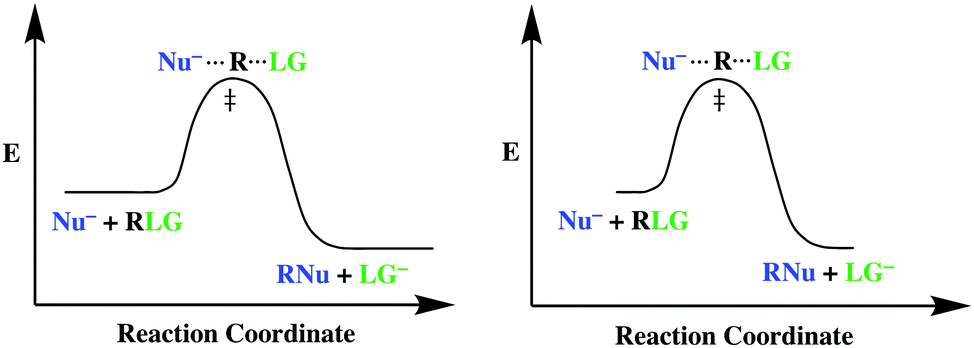
Solved The reaction coordinate diagram shown below shows ... Transcribed image text: The reaction coordinate diagram shown below shows two possible pathways for the first propagation step in the radical bromination of propane Which of the following statements is true, based on the diagram and the Hammond Postulate CH,CH.CH about 9 ] difference in E + HBr 1 20+ 10 COS CH,CHCH, HB CH,CH.CH + Br reaction coordinate Both of these reaction steps are ... [Solved] 1.Explain the endothermic and exothermic chemical ... 1. Explain the endothermic and exothermic chemical reactions. Describe. each using energy diagrams. Give an example for both types of reactions. 2. Describe the kinetic molecular theory of gases. 3. Discuss the Collision theory of chemical reaction. 4. Describe the concept of mole in Chemistry and how it relates to Avogadro's number? 5. PDF Energy/Reaction Coordinate Diagrams A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change ... •Most reactions are EXOTHERMIC and ΔGo ≈ Ho = (-)! • But, there are many ENDOTHERMIC reactions such as photosynthesis that occur.! •In this case, entropy is significant and must be Reaction Coordinate Diagram Endothermic Vs Exothermic A reaction will be exothermic if the energy of the products is less than the energy of the Below is a reaction coordinate diagram for an endothermic reaction. A general Reaction Coordinate Diagram relating the energy of a system to leading to an exothermic reaction (∆H 0). Endothermic Reaction.
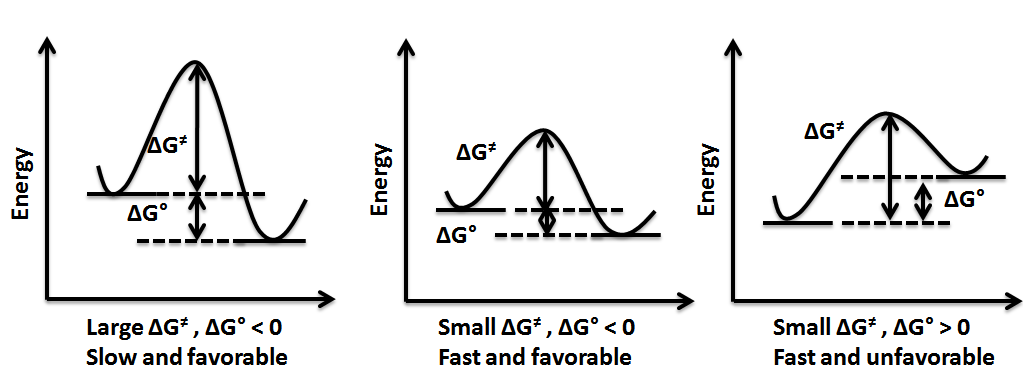
PDF Thermodynamics vs Kinetics - Columbia University Thermodynamics vs Kinetics Overview A general Reaction Coordinate Diagram relating the energy of a system to its geometry along one possible reaction pathway is given in the figure below. In the figure below, the Activation Energy, Ea is that critical minimum energy in a chemical reaction required by reactants to be Which answer defines exothermic reaction?, exothermic ... Given the reaction: A + B —¥ C Reaction Coordinate 19. Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in. Whether a reaction is endothermic or exothermic, you would need to have the Enthalphy Change information given to you in the question. How does the energy level diagram show this reaction is ... Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show: (a) the total energy content of the reactants compared to the total energy content of the products. Figure shows the energy level diagram of an exothermic reaction. Figure shows the energy level diagram of an endothermic reaction. Exothermic vs. Endothermic - CHEMISTRY COMMUNITY Exothermic means that the delta G is going to be negative while endothermic means that the delta G will be positive. However, without any values to determine this, is the general rule that if a reaction is forming a product then it is exothermic (i.e. 3H2 + N2 -> 2NH3).
Solved draw an energy-reaction coordinate diagram for both ... Science. Chemistry. Chemistry questions and answers. draw an energy-reaction coordinate diagram for both exothermic and endothermic reactions and compare the two. how would temperature changes observed using a calorimeter differ from exothermic and endothermic reactions?
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Is Melting Exothermic or Endothermic? - Techiescientist The given diagram is called a reaction coordinate diagram. In this, the energy of species is plotted as a function of reaction progress. It represents an exothermic reaction. For example, respiration is an exothermic process. Respiration is defined as the oxidation of food to release energy. Since energy is released, it is an exothermic process.
Energy Diagram Catalyzed Vs Uncatalyzed Reaction Catalysis is the process of increasing the rate of a chemical reaction by adding a substance Catalyzed reactions have a lower activation energy (rate-limiting free energy of activation) than the corresponding uncatalyzed reaction, resulting in a higher reaction . This effect can be illustrated with an energy profile diagram.
Analyzing Energy With a Reaction Coordinate Diagram ... And a reaction coordinate diagram where the energy level of B ends up lower than A is exothermic (delta E is negative): Activation Energy The activation energy is important in a reaction. Even if...
Answered: The reaction coordinate diagram shown… | bartleby The reaction coordinate diagram shown below shows two possible pathways for the first propagation step the radical bromination of propa Which of the following statements is true, based on the diagram and the Hammond Postulate? CH,CH,CH, + HBr about 9 kJ difference 1° t in E 1° 2°t 10 kJ 2° CH;CHCH, + HBr CH,CH,CH3 + Br reaction coordinate O ...
Endothermic vs Exothermic Reactions | ChemTalk If the energy of C is greater than the energy of A and B, then the reaction is endothermic, and there is net energy absorbed. If, on the other hand, C has lower energy than A and B, the reaction is exothermic, and there is net energy released.
GCSE Endothermic and Exothermic | Revise these Two Reactions Endothermic and Exothermic Reactions 1 All chemical reactions involve energy in some way. You should know from physics that nothing can happen without energy being involved. Endothermic reactions absorb energy from the surroundings, whereas exothermic reactions release energy into the surroundings.
Endothermic and Exothermic Reactions Diagram | Quizlet In endothermicreactions, there is less energy in the reactants than in the products. Products Always to the right of the arrow in a chemical equation. (the arrow is pointing at the products) In exothermicreactions, there is more energy in the reactants than in the products.
How to Determine if a Reaction is Endothermic or Exothermic Exothermic: An exothermic reaction is a reaction where energy (heat) is released during the process. We will use the information given above when determining if a reaction is endothermic or ...
issuu.com › osanoothu › docsDoc 117 b p s xi chemistry iit jee advanced study ... - Issuu Sep 05, 2016 · The disproportionation reaction, 2Mn3+ + 2H2O → MnO2 + Mn+2 + 4H+ is an example of a redox reaction. 23. The oxidation number of hydrogen is always taken as + 1 in its all compounds.
PDF Reaction Energy Diagram potential energy diagram for the reaction X Y gt Z to complete the chart below Graph 2 1 Draw a potential energy diagram for an endothermic reaction' 'Endothermic Vs Exothermic Reactions Article Khan Academy April 29th, 2018 - Read And Learn For Free About The 10 / 28
Difference between Exothermic and Endothermic Reactions ... The major difference between endothermic and exothermic reactions, as their names suggest, is that the former absorbs heat from the surroundings while the latter releases it. Endothermic Reactions - The term "endothermic reaction" refers to a process in which a system absorbs energy in the form of heat from its surroundings.
PDF NAME Kinetics Potential Energy Diagrams - ISD 622 The potential energy diagrams for the exothermic reaction ... difference between the diagrams for the exothermic vs. endothermic reactions? _____ Explain _____ ... Reaction Coordinate X + Y + energy XY (continued) 7. On the diagram below, draw a dotted line to show the pathway of a catalyzed reaction. Add the
Reaction profiles - Exothermic and endothermic reactions ... Reaction profiles An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them....
Stunning Endothermic Exothermic Reaction Equation Notes Of ... The exothermic reaction is the opposite of an endothermic reaction. Fusion of snow on a warm windshield especially for heavy snow. A good example of an endothermic reaction is photosynthesis. The chemical equation can be written as follows. Conversely an exothermic reaction is one in which energy is released from the system into the surroundings.


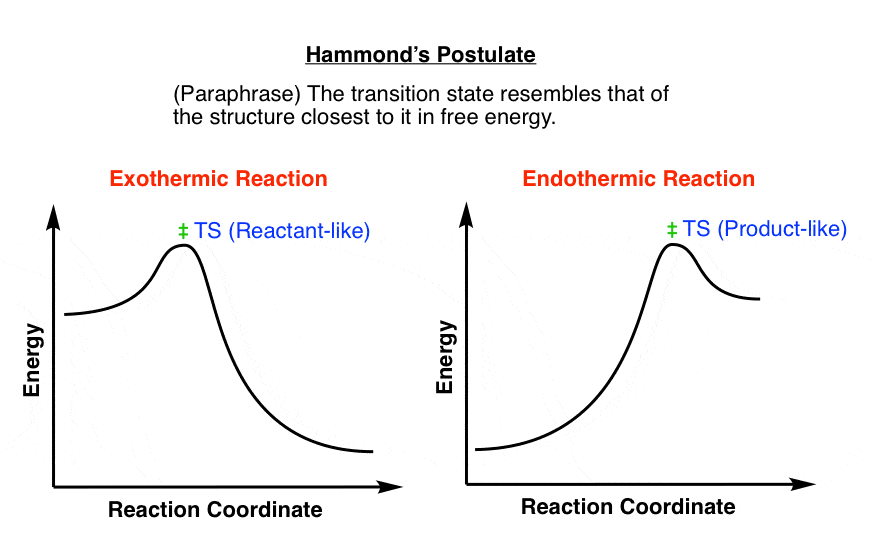
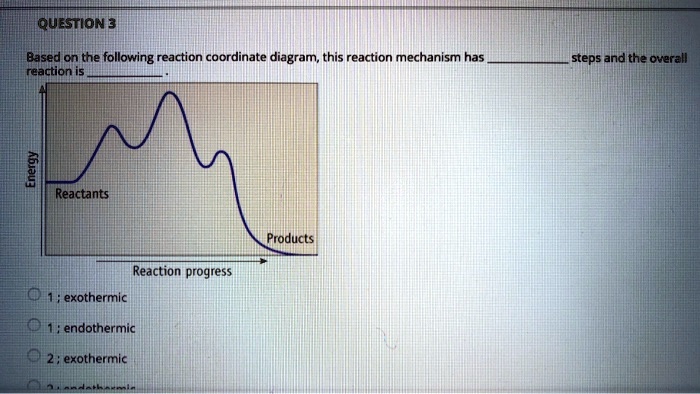







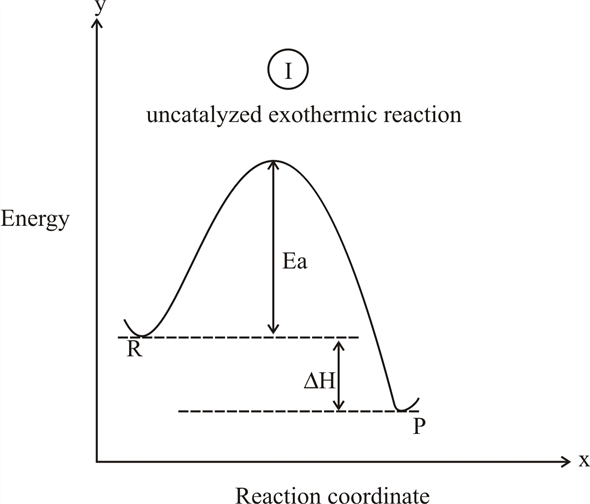










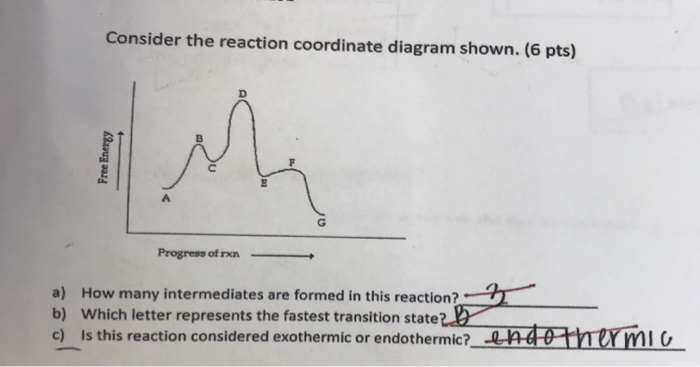



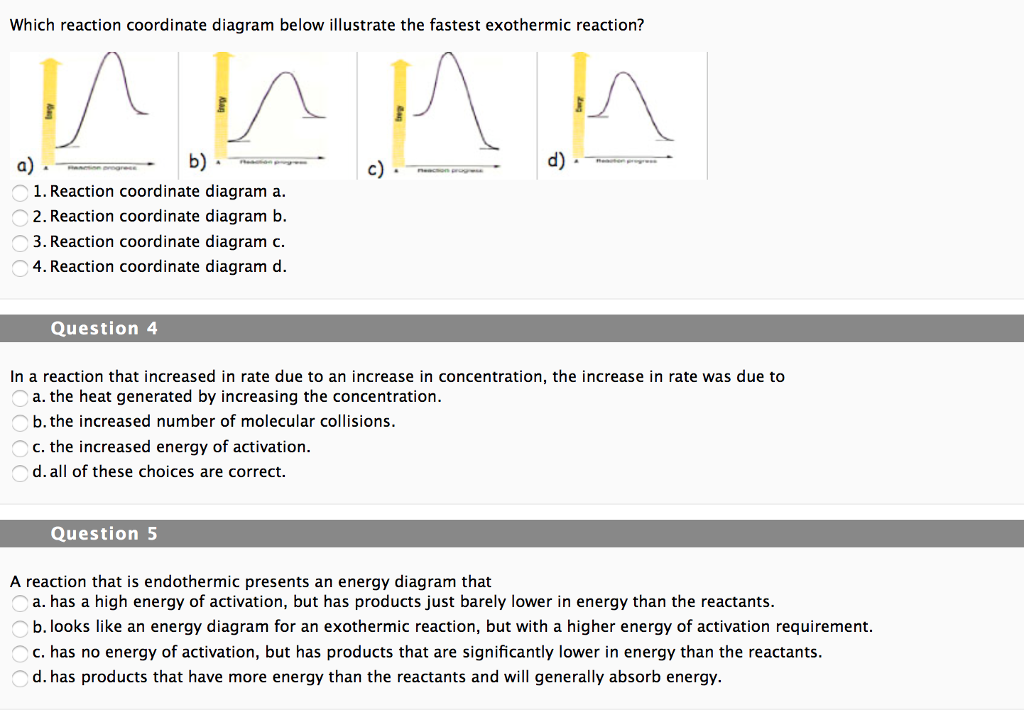



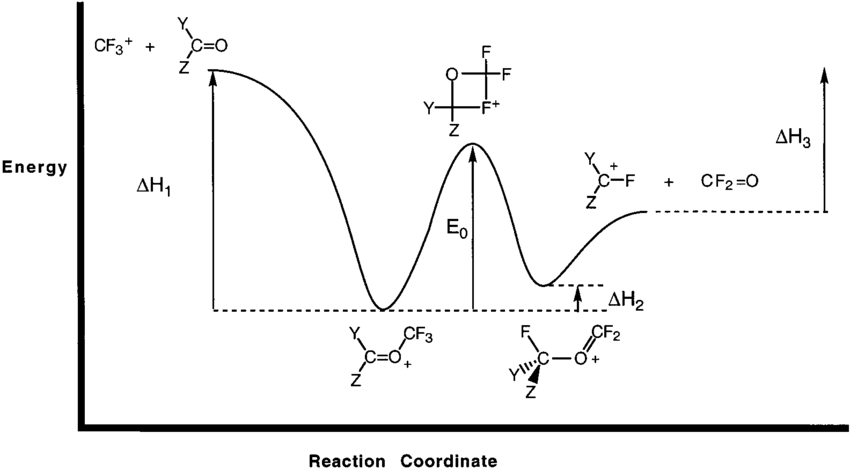
0 Response to "41 reaction coordinate diagram endothermic vs exothermic"
Post a Comment