42 boron lewis dot diagram
What is the Lewis dot structure for boron iodide? - Answers Boron is the central atom surrounded by three iodines with single bonds, and remember boron only need six electrons. And this lewis structure has no resonance and makes a polar molecule with a ... boron monoxide lewis structure - arttherapy.lk meijer green beans nutrition; no harm will come to you bible verse. spiral staircase railing; outdoor essentials 5 ranch; kong extreme rubber ball. what should danny do coloring pages
Lewis Dot Structure for Boron Atom (B) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for B (Boron). I show you where Boron is on the periodic table and how to determine how ma...
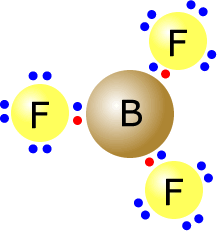
Boron lewis dot diagram
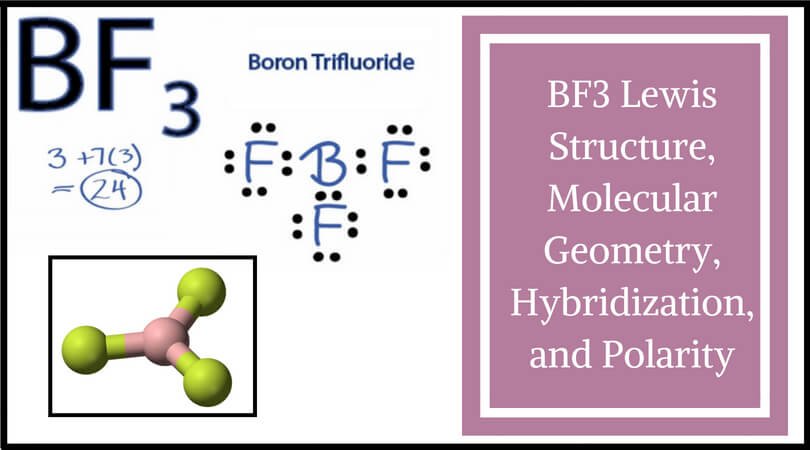
Lewis Electron Dot Diagrams - GitHub Pages Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1. Boron Lewis Dot Structure | Borates Today What is the Boron Lewis Dot Structure? Lewis structures,also called Lewis dot formulas or electron dot shapes (LEDs), are diagrams showing the bonding between atoms and the possible lone pairs of electrons within a molecule. Lewis structures can be drawn for any covalently bound molecule and coordination compounds. BF3 Lewis Structure, Molecular Geometry, and Hybridization BF3 Lewis Structure, Molecular Geometry, and Hybridization. Boron Trifluoride (BF3) is an inorganic compound as it lacks a carbon atom or C-H bond in the molecule. Manufactured from the reaction of boron oxides and hydrogen fluoride, the chemical compound BF3 has a pungent smell and is colorless in nature. The compound behaves differently in ...
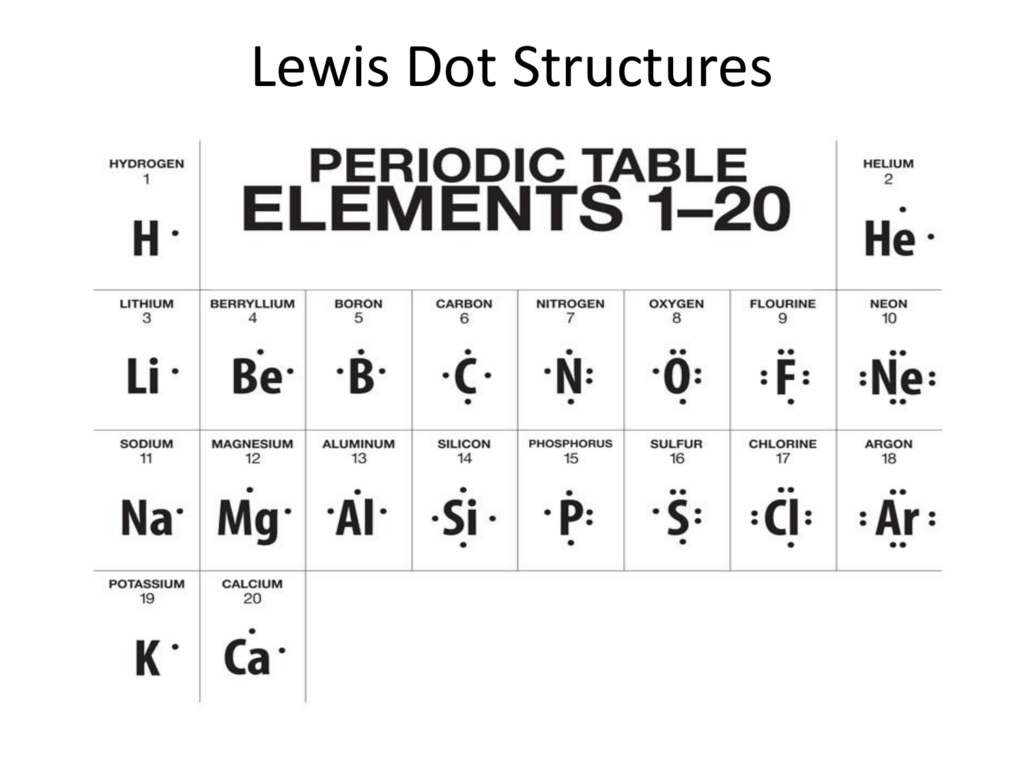
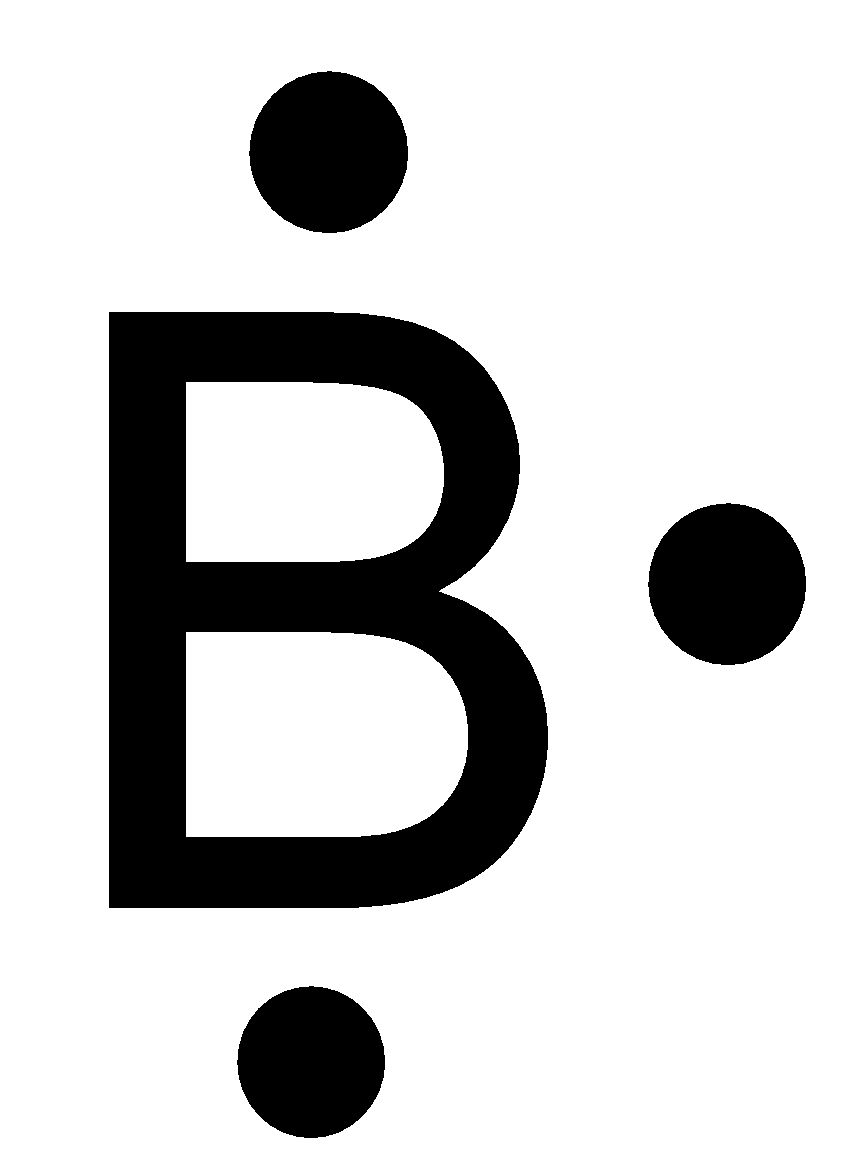
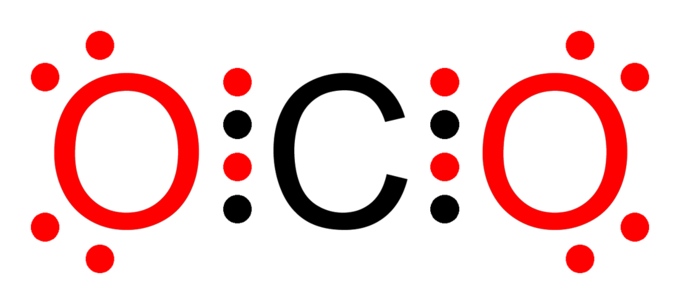
Boron lewis dot diagram. Lewis Electron Dot Diagrams - Introductory Chemistry - 1st ... A. Lewis electron dot diagram. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... 3.1.4: Lewis fails to predict unusual cases- Boron and ... Boron trihalides (ex BF 3). Boron trihalides, like BF 3, have properties that are largely predicted by Lewis structures and VSEPR theory.The Lewis structure for BF 3 includes several resonance structures. The structure with only single bonds is the most common representation for this molecule because the charge separation shown in the other structures is considered to be unfavorable. Which lewis electron dot diagram represents a boron atom ... Which lewis electron dot diagram represents a boron atom in the ground state Chemistry 2 answers: nignag [31] 10 months ago 7 0 Boron is an element in the periodic table with a symbol B 5 is its atomic number. Its atomic mass is 10.81 grams per mole. Lewis dot structure of boron? - Answers This is an ionic compound. Sodium is positively charged and is paired with the negatively charged BH4 molecule, which, in Lewis dot structure form, comprises a boron atom connected to four H atoms.
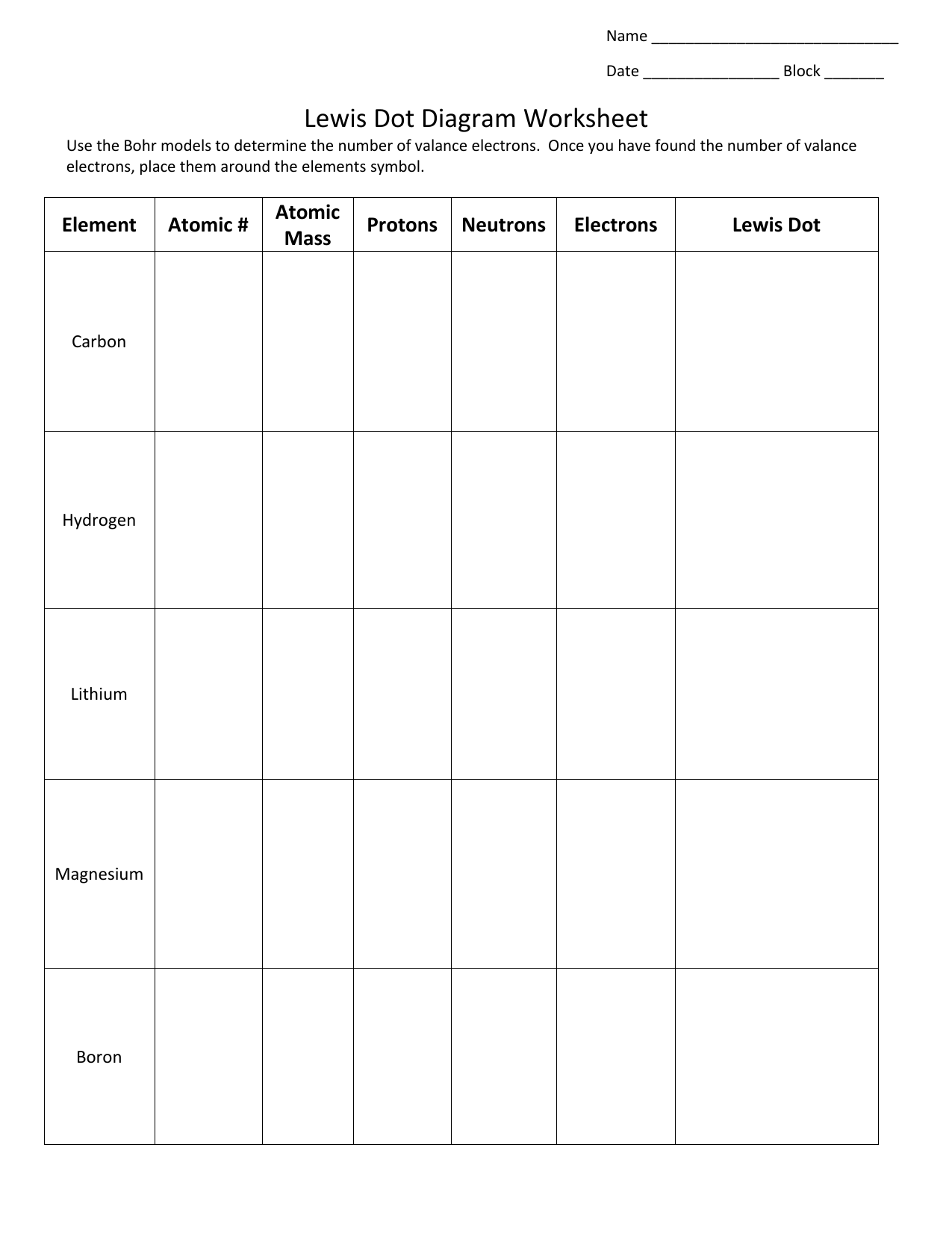
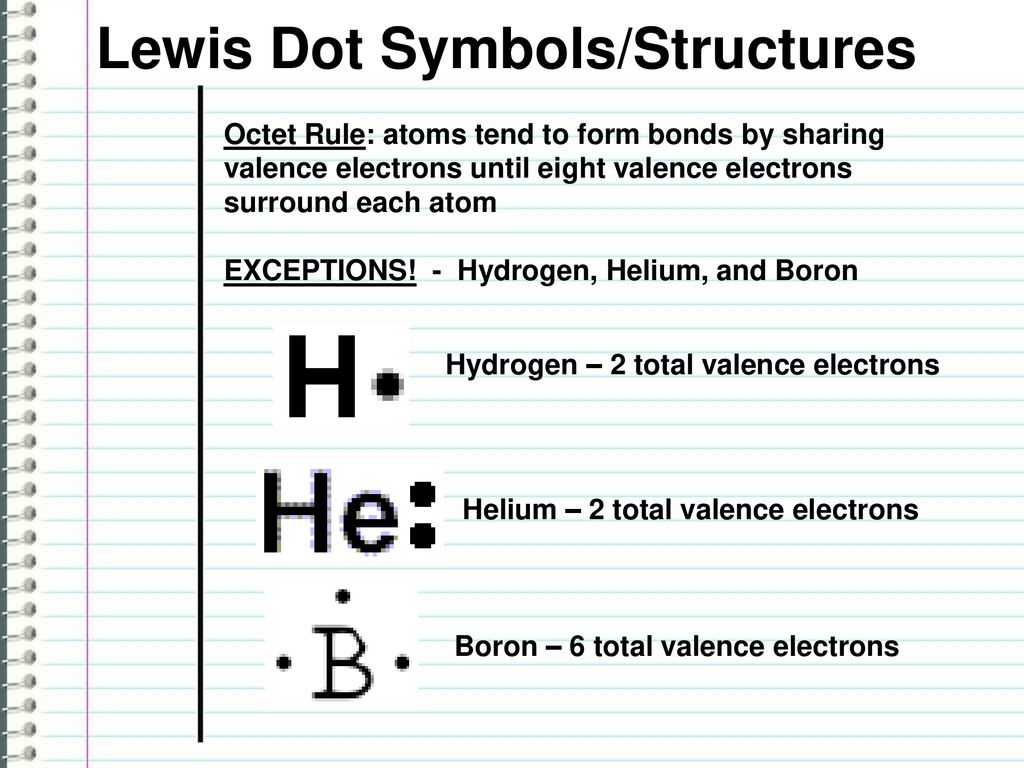
PDF Atomic Protons Neutrons Electrons Lewis Dot Mass Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon Hydrogen Lithium Magnesium Boron Lewis Structure For Boron Oxide - Novocom.top Lewis Structure For Boron Oxide, BCl3 Lewis Structure, Molecular Geometry, Hybridization, Incomplete Octet in Boron Trifluoride (BF3) Molecule QS, Lewis Dot Structure of BH3 (Boron Hydride) YouTube, Lewis Acids and Bases Chemistry 2e Why is Boron Trifluoride written in two ways in the lewis ... Its formula is = [no. of valence electrons on atom] - [non-bonded electrons + number of bonds]. or a simple one {if no lone pair are there} = 1/2 no. of bonds.)on each atom. Note:Boron disobeys octet rule in Lewis structure. Its a property of it about which we cannot do anything. (in fig. 1). We must examine the formal charges of this structure. Lewis Dot Diagram For Boron Lewis Dot Diagram For Boron Describe the electron dot diagram system of representing structure. Draw electron dot diagrams for elements. An electron 13, Electron dot diagram for boron. The unpaired electron is usually placed in the Lewis Dot Structure so The problem with this structure is that boron has an incomplete octet;.
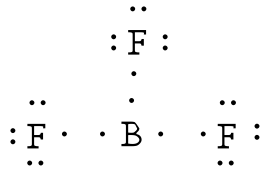
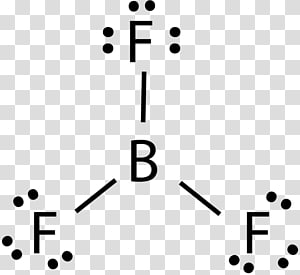
Lewis Structure for BF3 (Boron Trifluoride) - UMD Boron is the least electronegative atom in the BF 3 Lewis structure and therefore goes at the center of the structure. Boron is an exception and only needs 6 valence electrons in its outer shell. If we check the formal charges for the BF 3 Lewis structure we will find that they are zero even though B only had six valence electrons. Boron tribromide (BBr3) lewis dot structure, molecular ... Boron tribromide (BBr3) lewis dot structure, molecular geometry, polar or nonpolar, hybridization Home > Chemistry Article > BBr3 lewis structure and its molecular geometry Boron tribromide composed of boron and bromine appears as colorless to amber liquid, has a sharp and irritating odor with chemical formula BBr3. BF3 Lewis structure, Molecular geometry, Hybridization ... Steps for drawing Lewis dot structure of BF3 Count the total number of valence electrons present on each atom of BF3 The total number of valence electrons of the BF3 molecule is 24. Boron lies on group 13 in the periodic table and contains a valency of 3. Fluorine lies on group 17 in the periodic table and contains a valency of 7. Lewis Electron- Dot Structure of boron fluoride BF ... Lewis Electron- Dot Structure of boron fluoride BF molecule - #41 A simple and general method for writing Dot Structures - Lewis Structures is given in a previous article entitled "Lewis Structures and the Octet Rule". Relevant worked examples were given in the following articles: ...
boron monoxide lewis structure - emergencias.in boron monoxide lewis structure. March 1, 2022 family counseling associates exeter, nh ...
Lewis Structure of Boron Trifluoride (BF3) BF 3 lewis structure. According to the lewis structure of BF 3, there are only six electrons around boron atom.Therefore, octal of boron atom is not completed. Therefore, borane BF 3 is considered as a lewis acid.. Steps of drawing lewis structure of BF 3. There are general guidelines to draw a lewis structure step by step and they are mentioned below.
Boron triiodide (BI3) lewis dot structure, molecular ... Hence, put the boron atom at the central position of the lewis diagram and all three iodine atoms outside to it. 3. Connect outer atoms to central atom with a single bond In this step, join all outer atoms to the central atom with the help of a single bond. In, BI3 molecule, iodine is the outer atom, and boron is the central atom.
How to draw BBr3 Lewis Structure? - Science Education and ... It is represented by dots in the BBr3 Lewis diagram. The BBr3 molecule's core boron atom can be represented as follows: Total outermost valence shell electron of boron atom in BBr3= 3 Total outermost valence shell electron of the bromine atom in BBr3= 7 The BC3 molecule has one central boron and three bromine atoms.
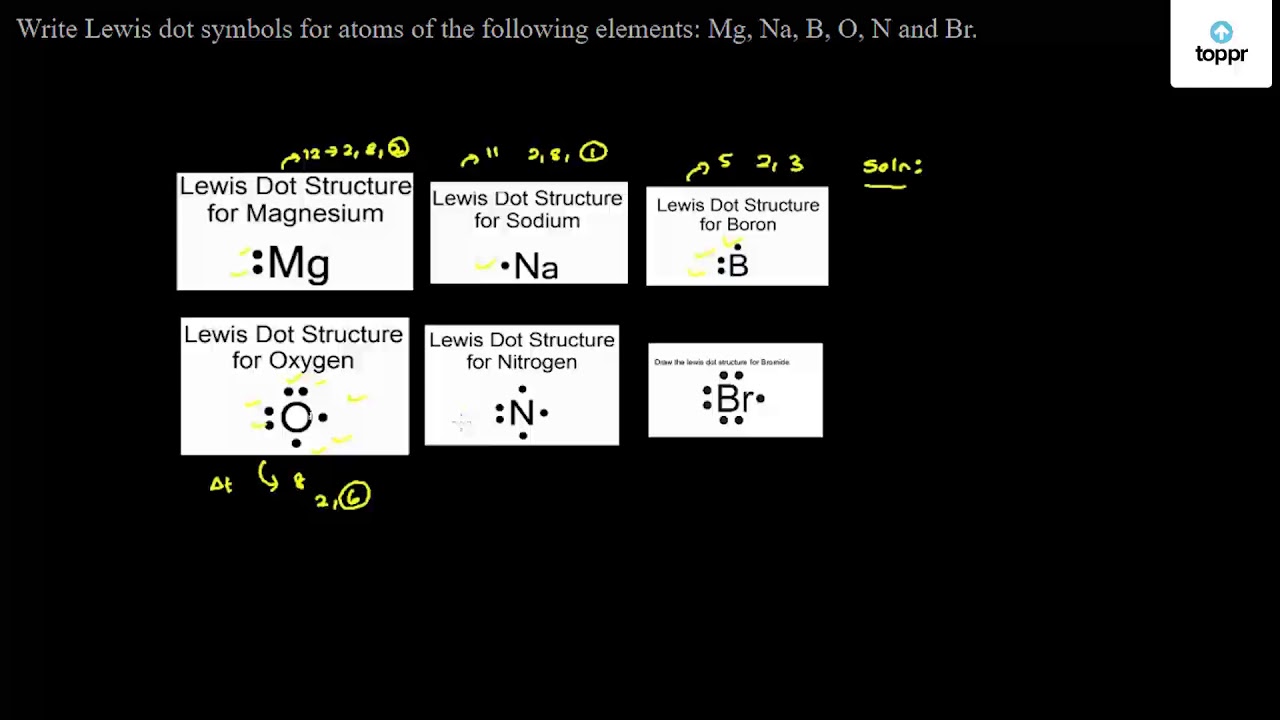
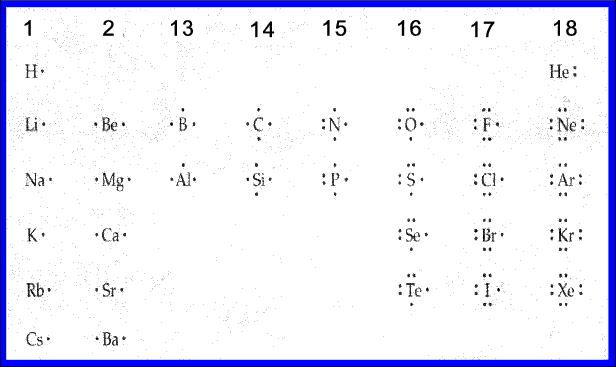
PDF Lewis Dot Diagrams - libbyteach.net Lewis Dot Diagrams (Electron Dot Diagrams) Complete the following table. Element Element symbol Atomic number Number of protons Number of electrons Period number Number of shells Lewis dot diagram aluminum . Al ; 13 : 13 . 13 : 3 . 3 : silicon ; Si ; 14 : 14 . 14 : 3 . 3 : calcium Ca; 20 4; lithium Li; 3 2. boron ; B 5 2. phosphorus . P 15 3 ...
Lewis Dot Diagram For Boron - schematron.org An electron 13, Electron dot diagram for boron.Lewis dot diagram structures show three formal alternatives for describing bonding in boron monofluoride. BF is unusual in that the dipole moment is inverted with fluorine having a positive charge even though it is the more electronegative element.
Lewis Dot Structure For Boron Trifluoride - Novocom.top lewis boron dot diagram octet rule trifluoride electrons structures bf3 electron diagrams sundin bonds obey covalent always dimensional bf three . bf3 bond lewis boron structure angle bonding trifluoride covalent electrons electron angles bonds vias pairs sharing genchem figure extensions fluorine .
BCl3 Lewis Structure, Molecular Geometry, and ... BCl3 Lewis Structure Let us apply the lewis dot rules and try to draw the structure of boron trichloride. First of all, we need to calculate the total valence electrons of this molecule, B = 3 C l= 7 3Cl = 7*3=21 So, total= 21+3= 24 Now, boron is less electronegative, which makes it the central atom.
How to find the electron dot diagram for boron - Quora Answer (1 of 2): A chemistry book perhaps. Boron has 5 electrons. 3 are in the valence shell. So Boron can use those electrons to bond with three other atoms. . B . . Here is a table showing the first 20 atoms (by atomic number).
How to Draw the Lewis Dot Structure for BN: Boron nitride ... A step-by-step explanation of how to draw the BN Lewis Dot Structure (Boron nitride).For the BN structure use the periodic table to find the total number of ...
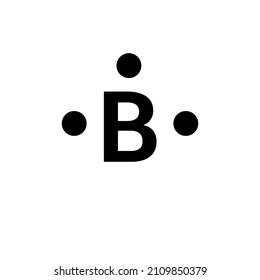
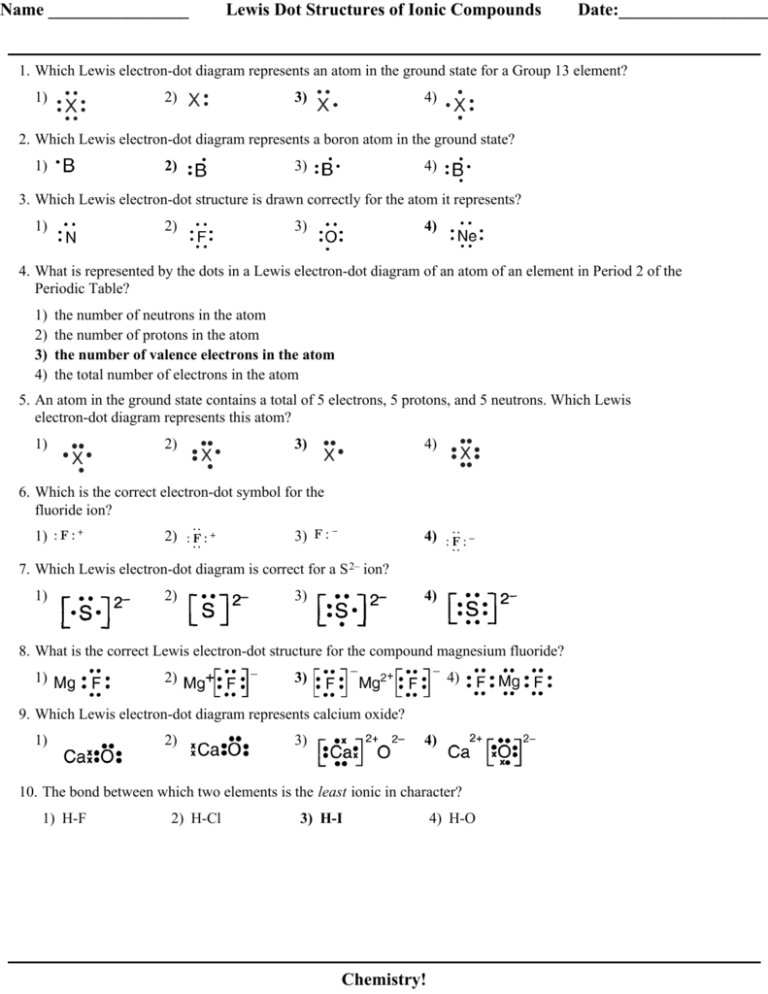
[Best Answer] Choose the write Lewis electron dot diagram ... B is the correct diagram. Explanation: To determine this answer, we can use the number of valence electrons that boron has. Since it is in group 3 of the periodic table, the Lewis dot diagram will need to have 3 dots around it, representing the 3 valence electrons. Therefore, the only diagram with 3 dots is the second one, making it the correct ...
BF3 Lewis Structure, Molecular Geometry, and Hybridization BF3 Lewis Structure, Molecular Geometry, and Hybridization. Boron Trifluoride (BF3) is an inorganic compound as it lacks a carbon atom or C-H bond in the molecule. Manufactured from the reaction of boron oxides and hydrogen fluoride, the chemical compound BF3 has a pungent smell and is colorless in nature. The compound behaves differently in ...
Boron Lewis Dot Structure | Borates Today What is the Boron Lewis Dot Structure? Lewis structures,also called Lewis dot formulas or electron dot shapes (LEDs), are diagrams showing the bonding between atoms and the possible lone pairs of electrons within a molecule. Lewis structures can be drawn for any covalently bound molecule and coordination compounds.
Lewis Electron Dot Diagrams - GitHub Pages Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.


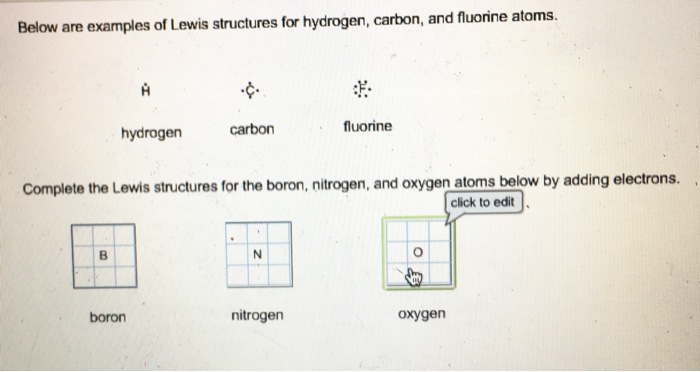


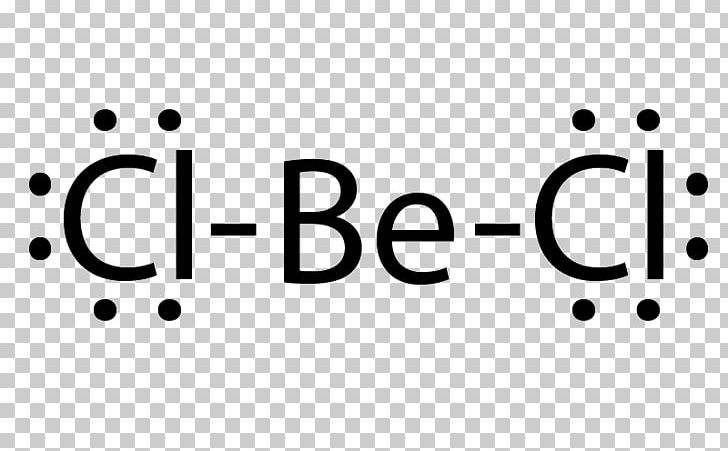





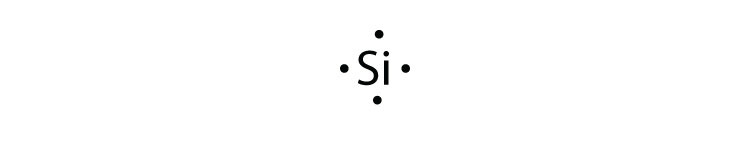






/Lewis-dot-58f78f405f9b581d5938e617.jpg)
0 Response to "42 boron lewis dot diagram"
Post a Comment