42 lewis dot diagram for n2
NO Lewis Dot Structure | Science Trends Lewis diagrams contain 3 basic elements: symbols that represent individual atoms, dots that represent electrons, and unbroken lines that represent shared electron 2NO + heat → N2 + O2. Nitric oxide is also one of the main causes of acid rain. Nitric oxide that is released in the atmosphere will react with... Based on the Lewis electron-dot diagram that you drew, is... - Brainly.in mintu78945 mintu78945. No, N₂ is not a polar molecule. Explanation: Polarity in a molecule develops due to electronegativity differences between atoms of that molecule. N₂ constitute 2 N atom bonded by 3 covalent bonds. Each N atom has a lone pair. Both N atoms are identical and equivalent.
What is the Lewis Structure for N2 (nitrogen gas)? - Quora Following is the Lewis dot cross structure for Nitrogen (N2) In N2 Lewis structure,two nitrogen atoms has shared six valence electrons and every nitrogen atom has one lone To draw the N2 Lewis structure , we have to find out the valence electrons of nitrogen first.We express valence electrons as...

Lewis dot diagram for n2
Lewis Dot Diagrams of the Elements - STEM Sheets Lewis dot diagrams are a visual representation of the valence electrons on an atom of each individual element. These diagrams are used to draw Lewis dot structures, also know as electron dot structures or Lewis dot formulas, of compounds. PDF Lewis dot diagrams (structures) for atoms and ions predicting... Draw Lewis dot structures for each of the following atoms: Aluminum. Determine the common oxidation number (charge) for each of the following ions, and then draw their Lewis Dot Structure. Don't forget to show brackets and charge on your LDS for ions! What is the Lewis dot diagram for N2? - Answers Best Answer. Copy. N2 is nitrogen gas, and is in group 5 therefore Add your answer: Earn +20 pts. Q: What is the Lewis dot diagram for N2?
Lewis dot diagram for n2. Lewis Electron Dot Diagram - Concept - Chemistry Video by Brightstorm Lewis Electron Dot Diagrams are used to visually depict bonding by representing valence electrons as dots surrounding an elemental symbol. These dots can be on any of the four sides of the symbol, each side representing a different orbital (1 s orbital and 3 p orbitals). Lewis Dot Diagrams of the Elements Lewis Symbols. Electron Configuration into Shells. The second shell, associated with principal quantum number n=2, can have a maximum of 8 electrons and corresponds to the second period of the periodic table. How to Draw the Lewis Dot Structure for N2: Nitrogen Gas... - YouTube A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find... PDF Microsoft Word - st | 9. Lewis Dot Diagrams for Bigger Molecules Draw the Lewis dot diagram for hydrochloric acid, HCl; use the Periodic Table and the instructions above. The biggest shell is also called the valence shell or the bonding shell. For example, water is H2O, and oxygen has 8 electrons - 2 electrons in the first shell and 6 electrons in the second shell.
chemistry-lewis dot diagrams A Lewis structure or Lewis dot diagram, represents the bonds formed between two non-metal atoms as they share electrons. These diagrams show only the valence electrons of each atom as they are distributed amongst the bonded atoms. Drawing such diagrams is a great start to understanding how... 4.2: Lewis (Electron-Dot) Symbols - Chemistry LibreTexts Draw a Lewis electron dot diagram for an atom. Know the importance of Lewis dot in bonding. Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis dot symbols can be used to predict the number of bonds formed by elements in a compound. Lewis Structures (electron dot diagrams) Chemistry Tutorial Lewis Structures or electron dot diagrams for atoms, ions, ionic compounds and covalent compounds tutorial with worked examples for chemistry students. Electrons in the Lewis Structure (electron dot diagram) are paired to show the bonding pair of electrons. Lewis Structures: Learn How to Draw Lewis Structures | Albert.io See the following Lewis dot structure diagrams for a few covalent compounds. Lewis Dot Structures for drawing polyatomic ions are done very similarly to that of drawing individual atoms or covalent compounds.
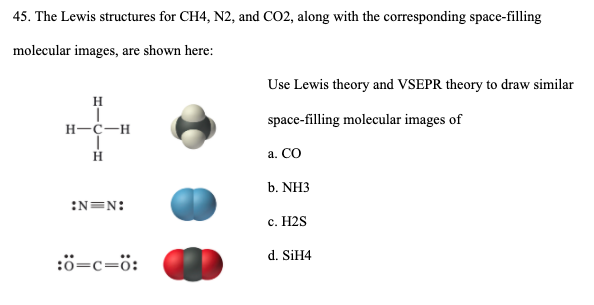
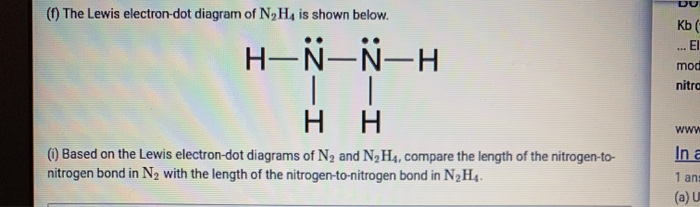
lewis dot diagram for nitrogen - Search Lewis Dot Diagrams, Atoms and Ions 1. Which Lewis electron-dot diagram represents a nitrogen atom in the ground state? The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's... PDF Lewis Diagrams 2 Lewis Diagrams For Covalent Bonding 3 Lewis Diagrams For Covalent Bonding 4 Forming Lewis Diagrams 5 Resonance 6 Beyond The Steps 7 This is in fact what happens. When we distribute the electrons according to our steps we arrive at: Lewis diagrams are electron dot pictures which give an... N2H4 lewis structure, molecular geometry, polarity, hybridization, angle Follow some steps for drawing the Lewis dot structure of N2H4. 1. Count total valence electron in N2H4. Because hydrogen only needs two-electron or one single bond to complete the outer shell. So, for N2H4, put away hydrogen outside and nitrogen as a central atom in the lewis diagram. Topic: Molecular Shape Do Now: Draw the Lewis Dot Diagram for... 4 Use the Lewis Structure Lewis structure is 2-D, but can help figure out 3-D shape. 5 Molecular Shape Shape determined by two factors: 1. # of unpaired e - around the central atom 2. total # atoms bonded to central atom. 6 It's important to classify electron pairs as bonding or nonbonding Electron pairs...
Drawing Lewis Dot Diagrams — bozemanscience Drawing Lewis Dot Diagrams. Mr. Andersen shows you how to draw Lewis Dot Diagrams for atoms and simple molecules.
Lewis structure - Wikipedia Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule.
Lewis Dot Diagram - Organic Chemistry | Socratic Lewis dot diagrams are a shorthand depiction of the bonds between several atoms and any unbonded electron pairs. Lines connect atoms to depict bonding and dots show the number of unbonded electrons still The Lewis dot diagram for the covalent bonding of chlorine, ( [Math Processing Error].
High School Chemistry/Lewis Electron Dot Diagrams - Wikibooks... This chapter will explore yet another shorthand method of representing the valence electrons. The method explored in this lesson will be a visual representation of the valence electrons. We will, as we observed in the previous lesson...
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry For example, the Lewis electron dot diagram for calcium is simply. Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. 1. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. 2. Is it necessary for the first...
Lewis Dot Diagram For Nitrogen - Free Diagram For Student Nitrogen is a chemical element with symbol n and atomic number 7. For diatomic nitrogen the lewis dot structure correctly predicts that the...
Lewis Dot Structure Example - Octet Rule Exception Lewis dot structures help predict molecular geometry. This example problem shows the steps to draw a structure where an atom violates the octet rule. Connect the atoms by single chemical bonds. The number of electrons to be placed is t-2n, where t is the total number of electrons and n is the number...
How to Draw Molecules and Chemical Bonds ... Just Like Lewis Dot... Lewis dot diagrams have lots of problems, and it is possible to do much, much better, with zero additional work, using hole-counting and related methods as discussed in section 2. 7.1 Hydrides and Other Successes. Of course the Lewis dot method is not entirely without merit.
Lewis Electron Dot Diagrams Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful.
3 Ways to Draw Lewis Dot Structures - wikiHow Drawing Lewis dot structures (also known as Lewis structures or Lewis diagrams) can be confusing, particularly for a beginning chemistry student. However, these structures are helpful in understanding the bonding and valence electron configurations of different atoms and molecules.
Lewis Dot Diagram For N - Wiring Site Resource Electron configuration into shells. More complicated versions can be used to show the bond between different atoms in a molecule.
PDF Microsoft Word - Chapter 5.docx Lewis dot structures for the first few non-‐metals. Silicon is similar to carbon, with "Si" replacing "C", phosphorous and arsenic are similar to nitrogen; sulfur, selenium, tellurium are similar to oxygen; chlorine, bromine, and iodine are similar to fluorine; and the Noble gases are similar to neon.
What is the Lewis dot diagram for N2? - Answers Best Answer. Copy. N2 is nitrogen gas, and is in group 5 therefore Add your answer: Earn +20 pts. Q: What is the Lewis dot diagram for N2?
PDF Lewis dot diagrams (structures) for atoms and ions predicting... Draw Lewis dot structures for each of the following atoms: Aluminum. Determine the common oxidation number (charge) for each of the following ions, and then draw their Lewis Dot Structure. Don't forget to show brackets and charge on your LDS for ions!
Lewis Dot Diagrams of the Elements - STEM Sheets Lewis dot diagrams are a visual representation of the valence electrons on an atom of each individual element. These diagrams are used to draw Lewis dot structures, also know as electron dot structures or Lewis dot formulas, of compounds.





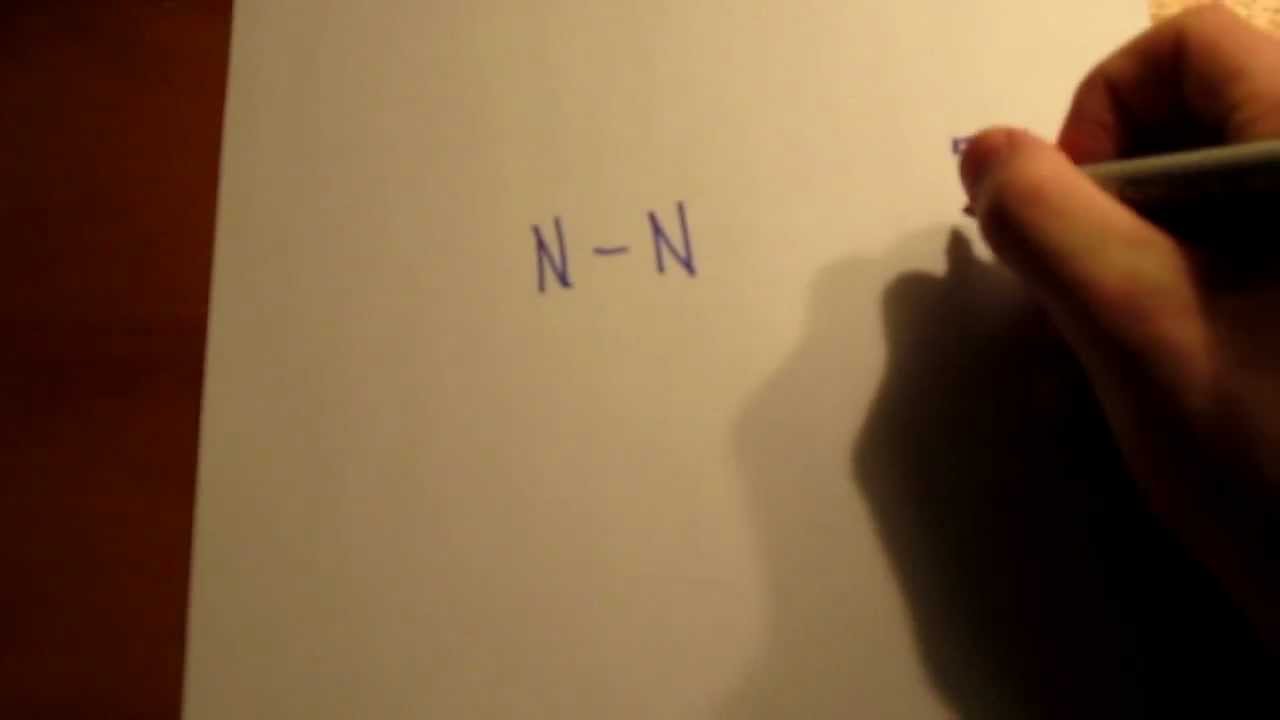
















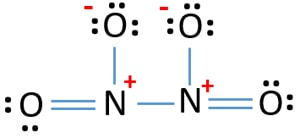

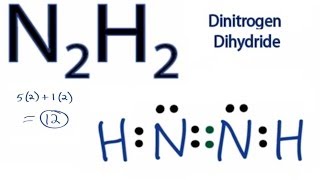




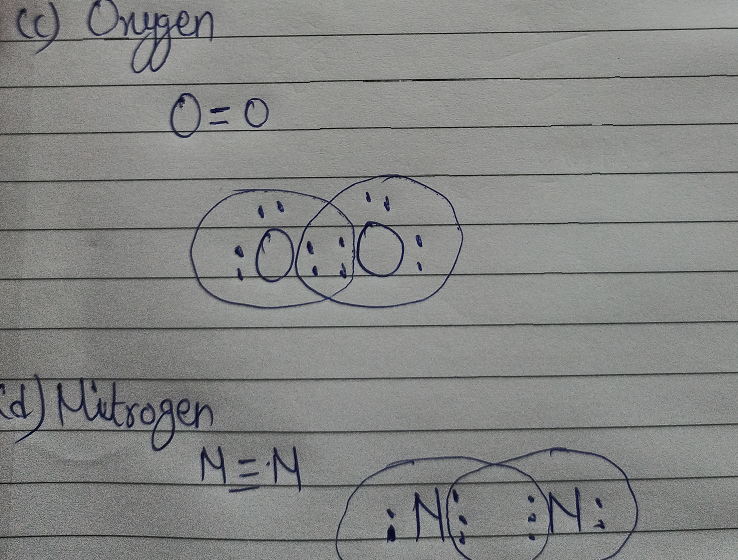
0 Response to "42 lewis dot diagram for n2"
Post a Comment