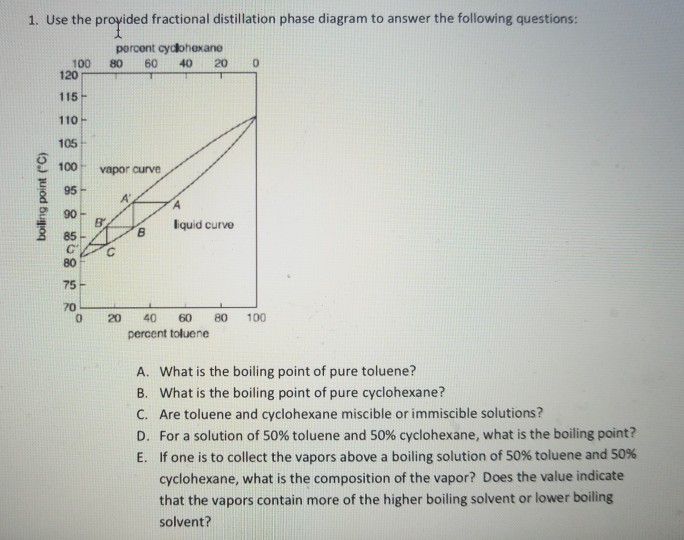
43 fractional distillation phase diagram
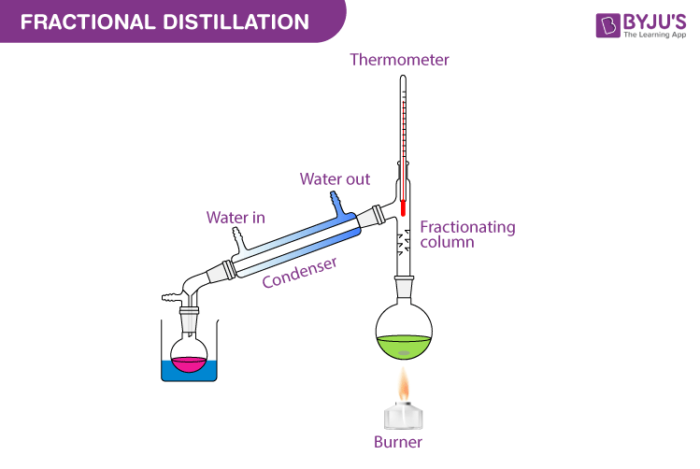
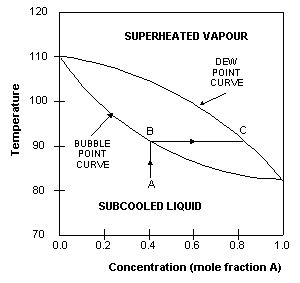
EXPERIMENT 7 - Distillation – Separation of a Mixture To understand the nature of simple distillation, fractional distillation and azeotropes we need to look at vapor/liquid diagrams for pairs of solvents. The graph below (Fig. 5) shows such a diagram for 2 solvents, A and B. A is the lower boiling material. The bottom of the graph shows the liquid state and the top of the graph shows the vapor state. chem.libretexts.org › Bookshelves › Physical_andFractional Distillation of Ideal Mixtures - Chemistry LibreTexts Aug 15, 2020 · Fractional distillation industrially There is no difference whatsoever in the theory involved. All that is different is what the fractionating column looks like. The diagram shows a simplified cross-section through a small part of a typical column. The column contains a number of trays that the liquid collects on as the vapor condenses.
KS4 Science - Teaching resources - Wordwall GCSE Biology Nervous system Reflex arc Labelled diagram. by Kac1. KS4 Biology Science. Simple Distillation Labelled diagram. by Sbenfield. ... Phase Change Graph - Heating Ice Labelled diagram. by Physicsmonkey2014. ... Fractional Distillation of Crude Oil Labelled diagram. by Clare11. KS4 Chemistry Science Crude Oil. B2 Topic 7 Ecology Quiz.

Fractional distillation phase diagram
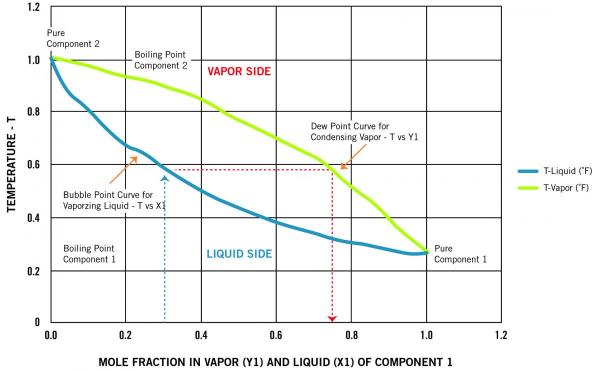
› qflab › 2020_21LABORATORY SESSION 6 Phase diagram: Boiling temperature ... temperature. These diagrams are needed when the aim is to separate the two liquids by fractional distillation. Figure 1 shows the phase diagram for an ideal solution. In a distillation experiment with constant pressure, the solution is heated and steam is extracted and condensed. The condensed liquid is richer in the more volatile component than in the original liquid. Fractional distillation repeats the boiling and condensation cycle several An innovative hollow fiber vacuum membrane distillation ... Feb 20, 2022 · The instantaneous phase separation during membrane-forming process induced the formation of a curved skinner layer on fiber lumen side which can be clearly seen from Fig. 4(b). In addition, it can be seen from the enlarged photos of the fiber outer skin layer ( Fig. 4 (c)) that a large number of sponge-like pores emerged in the outer skin layer ... › physical › phaseeqiafractional distillation of ideal mixtures of liquids Fractional distillation industrially There is no difference whatsoever in the theory involved. All that is different is what the fractionating column looks like. The diagram shows a simplified cross-section through a small part of a typical column. The column contains a number of trays that the liquid collects on as the vapour condenses.
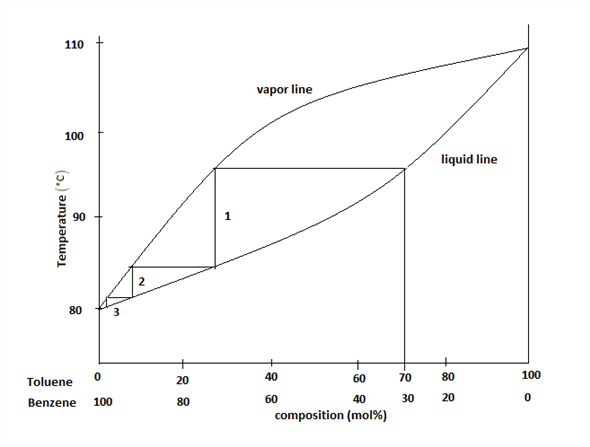
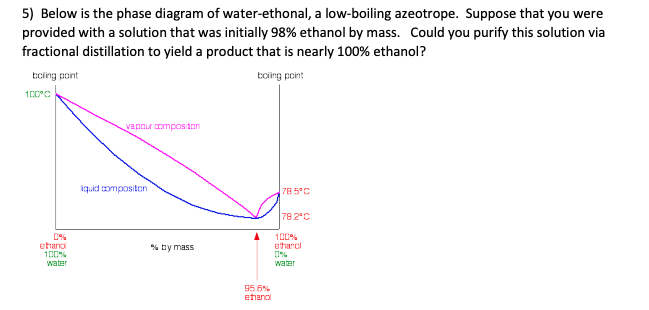
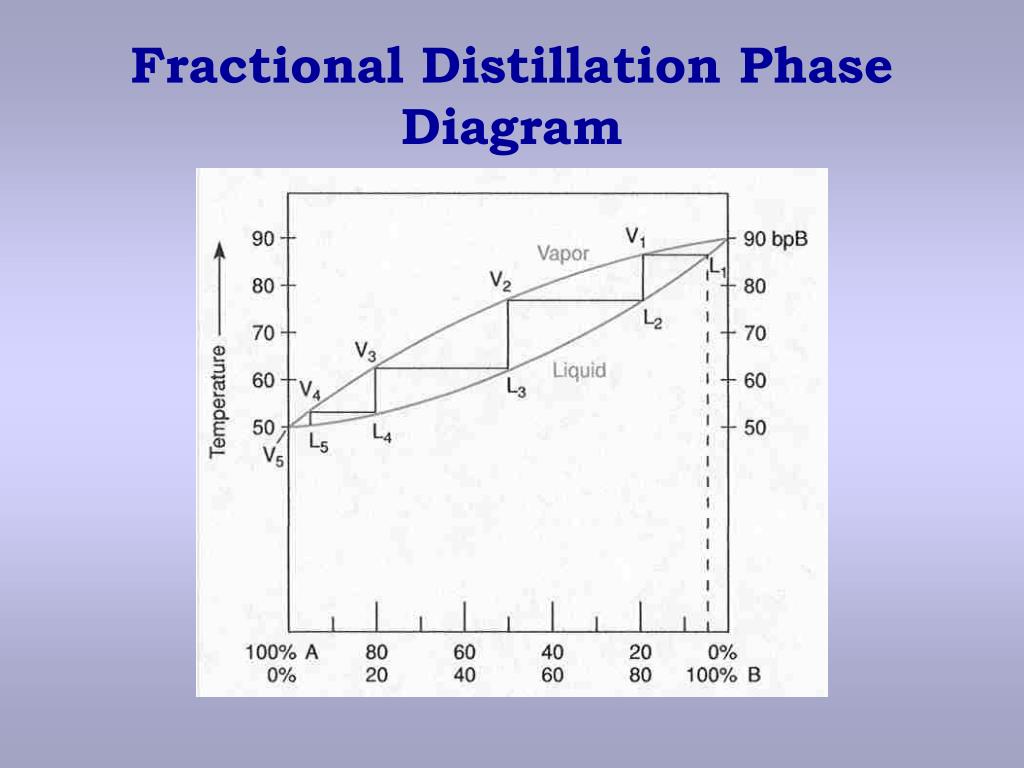
Fractional distillation phase diagram. Fractional distillation - New World Encyclopedia Previous (Fraction (mathematics)). Next (Framing (construction)). Fractional distillation is a special type of distillation designed to separate a mixture of two or more liquids that have different boiling points. simple distillation fractional distillation method of separating... Simple distillation and fractional distillation are two techniques used in the isolation and purification of liquid product from a chemical reaction. Revision notes on simple distillation diagram, fractional distillation apparatus explained, help when revising for AQA GCSE 9-1 chemistry, Edexcel GCSE... Fractional freezing - Wikipedia Fractional freezing is a process used in process engineering and chemistry to separate substances with different melting points. It can be done by partial melting of a solid, for example in zone refining of silicon or metals, or by partial crystallization of a liquid, as in freeze distillation, also called normal freezing or progressive freezing.The initial sample is thus fractionated ... Experiment 6: Fractional Distillation PowerPoint Presentation Fractional Distillation Phase Diagram. The arrows indicate a theoretical plate! Theoretical Plates Required to Separate Mixtures based on BP Boiling Point Difference Theoretical Plates Observations with maximum boiling azeotrope On the right side of the diagram: Compound B will distill (lowest bp).
Distillation - Wikipedia Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation. Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids). Dry distillation may involve chemical changes such as destructive distillation or cracking and is … Phase Diagrams For Fractional Distillation - Free Catalogs A to Z 6 hours ago Phase Diagrams Even with fractional distillation, our end product is likely to still have a small concentration of the other substance mixed in. One can calculate the concentration of a substance in the vapour by calculating the temperature at which the substance boils in a solution of a... Basic Steps to Fractional Distillation | Understanding Distillation Fractional Distillation Separates Substances Into Fractions, or Parts, Based on Their Various Boiling Points. See the Basic Steps and Learn How It Vapor reaching the top of the column (distillate) is collected into an industrial condenser (a big chiller), which cools the vapor back into a liquid, and... PDF Distillation fractionation (and hence fractional distillation). The processes of condensation and revapourisation take. place in the cooler parts of the distillation The vapour-liquid phase diagram for two liquids, X and Y, differing in boiling point by 7°C is shown above. (right). To obtain pure Y a large number of...
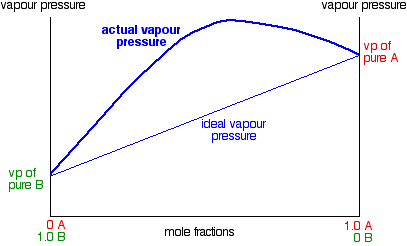
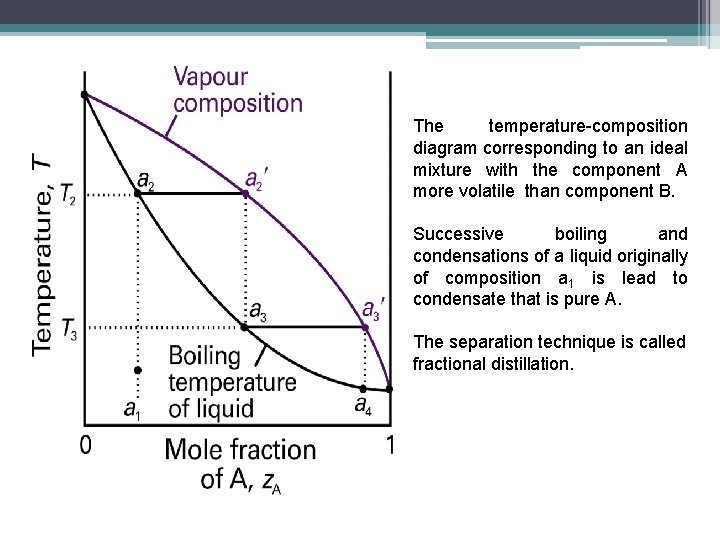
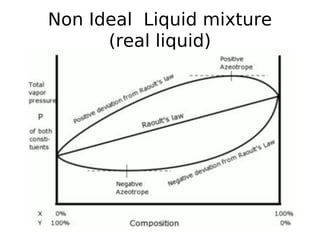
Fractional Distillation Phase Diagram. WWU -- Chemistry. Fractional Distillation Phase Diagram. The arrows indicate a theoretical plate! On the right side of the diagram: The azeotrope is the lower boiling compound, and it will be removed first. Pure ethanol will distill once the azeotrope has distilled. Separation by Fractional Distillation - GeeksforGeeks Fractional Distillation is used to separate miscible liquids that are volatile in nature. The boiling points of these liquids are close enough. One phase is the aqueous phase and the other phase is an organic solvent. This separation is based on the differences in the densities of the liquids. Chapter 8 Phase Diagrams In Fractional distillation, the boiling and condensation cycle is repeated successively. A maximum in a phase diagram may occur when favorable interactions between A and B molecules reduce the vapor pressure of the mixture below the ideal value. Raoult's Law and ideal mixtures of liquids - chemguide Using the phase diagram. The diagram is used in exactly the same way as it was built up. If you boil a liquid mixture, you can find out the temperature it boils at, and the composition of the vapour over the boiling liquid. ... The beginnings of fractional distillation.

09 :Fractional Distillation with Boiling point Diagram/Graph||Temperature composition phase diagrams
classes.kvcc.edu › chm220 › CHM220 Distillation LabDistillation Lab - Kalamazoo Valley Community College CHM220 Distillation Lab Page 4 of 7 Figure 5b Fractional Distillation Phase Diagram. The arrows indicate a theoretical plate. Each vaporization is represented by a horizontal line connecting the liquid composition curve to the vapor composition curve. Each condensation is represented by a vertical line connecting the
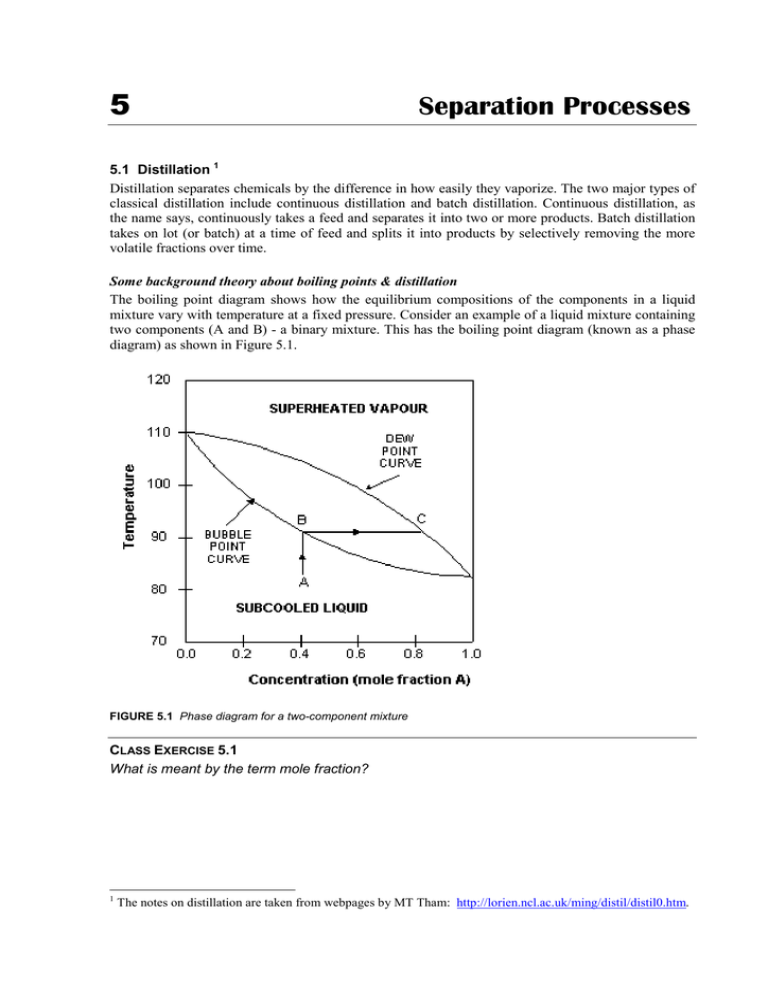
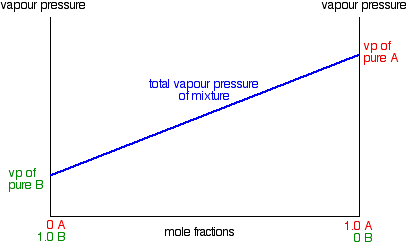
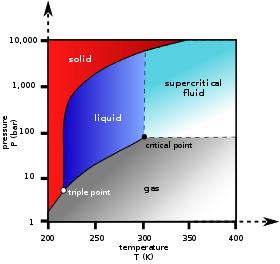
› Department › Chemical EngineeringMODULE 5: DISTILLATION 5.1.2. Phase Diagram For binary mixture phase diagram only two-component mixture, (e.g. A (more volatile) and B (less volatile)) are considered. There are two types of phase diagram: constant pressure and constant temperature. 5.1.3. Constant Pressure Phase Diagram The Figure 5.1 shows a constant pressure phase diagram for an ideal solution
PDF Phase Diagrams A phase diagram is actually a collection of solubility limit curves. The phase fields in equilibrium diagrams depend on the particular systems being depicted. Set of solubility curves that represents locus of temperatures above which all compositions are liquid are called liquidus...
Fractional Distillation So, a multi-stage Fractional Distillation technique will have to be employed instead. When a liquid mixture is in equilibrium with its vapor, the vapor It is much easier to vaporize a liquid in a Distilling Pot by raising the temperature of the liquid and boiling it. Our P vs. z Phase Diagram can be...
Crude Oil Distillation - an overview | ScienceDirect Topics A crude oil distillation plant has many components, e.g., crude oil furnace, distillation towers, and heat exchangers network. Fig. 16.1 illustrates a schematic diagram of the crude oil distillation system considered here. The system consists of two crude oil distillation units: the atmospheric distillation unit (ADU) and the vacuum distillation unit (VDU), two crude oil …
What is fractional distilation? - Quora Fractional distillation is a special type of distillation. designed to separate a mixture of two or more liquids. that have different boiling points. An example of a fractional distillation apparatus used in a laboratory. The diagram shows the use of a Liebig condenser and a conical flask as a receiving flask.
What Is Distillation? - Definition, Process & Apparatus ... Sep 22, 2021 · One way to speed up this process is to use fractional distillation. In this case, you need additional apparatus. The main piece of equipment is a fractionating column , which goes in the top of ...
Phase separation and fractional distillation 2.7 illustrates typical phase diagrams with a phase separation between the liquid and vapor phase. In a simple distillation the vapor is withdrawn and To separate two volatile liquids with a phase diagram as shown in Fig. 2.7 fractional distillation is used, i.e. the boiling and condensation cycle is...
Fractional Distillation - an overview | ScienceDirect Topics Fractional distillation of crude oil yields a product boiling between 30°C and about 200°C, known xw = the molecular fraction of the more volatile component in W. In the McCabe−Thiele Diagram The liquid phase is generally characterised by fractional distillation and measuring the properties of...
Fractional Distillation Definition and Examples Fractional distillation is used to purify chemicals and to separate mixtures to obtain their components. Gasoline and many other chemicals are produced from crude oil using fractional distillation. Crude oil is heated until it evaporates.
Fractional_distillation Fractional distillation Fractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds. The apparatus (the diagram represents a batch apparatus, as opposed to a continuous apparatus) is assembled as in the diagram.
Fractional distillation - Wikipedia Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate.
PDF Distillation of Liquids: Separation of 2-Propanol from Water by... Fractional distillation is used to separate liquid mixtures, soluble in each other with boiling point To complete the description of a distillation we must also describe what happens in the vapor phase. Fractional distillation accomplishes the same result. The difference is the presence of a fractionating...
Difference Between Fractional Distillation and Simple Distillation What is the difference between Fractional Distillation and Simple Distillation? Fractional distillation separates liquids with closer boiling points while.. When a mixture of liquids is heated, the components which have different boiling points go into gaseous phase at different instances.
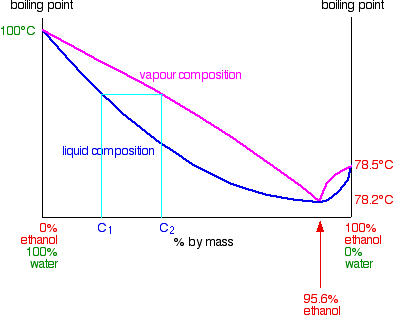
ALCOHOL DISTILLATION: BASIC PRINCIPLES, EQUIPMENT ... The previous relationships of alcohol-water mixtures hold true up to alcohol concentrations of about 95.6 percent. At this concentration, the two substances quit boiling separately (i.e., the alcohol in the vapor phase is no longer more concentrated than in the liquid phase), and fractional distillation no longer works.
Phase diagram, fractional distillation - Big Chemical Encyclopedia Phase diagram for a fractional distillation of an ideal two-component system. Figure 8.5 Vapor vs. liquid mole fractions (xy) phase diagram for methanol (a)-water (b) binary mixtures at a constant pressure of 1 atm. (a) xy diagram with 45° line indicated (b) stages in distillation at total reflux (c)...
Fractional distillation | Engineering | Fandom Fractional distillation is the separation of a mixture of miscible compounds by their boiling point, by heating to high enough temperatures. Main article: Oil refinery#operation. Distillation is the most common form of separation technology in the chemical industry.
Experiment 6: Fractional Distillation Reading Assignment... 13 Fractional Distillation Phase Diagram. 14 How many theoretical plates are need to separate a mixture starting at L? 15 Fractional Distillation Phase Diagram. The arrows indicate a theoretical plate! 16 Theoretical Plates Required to Separate Mixtures based on BP Boiling Point Difference...
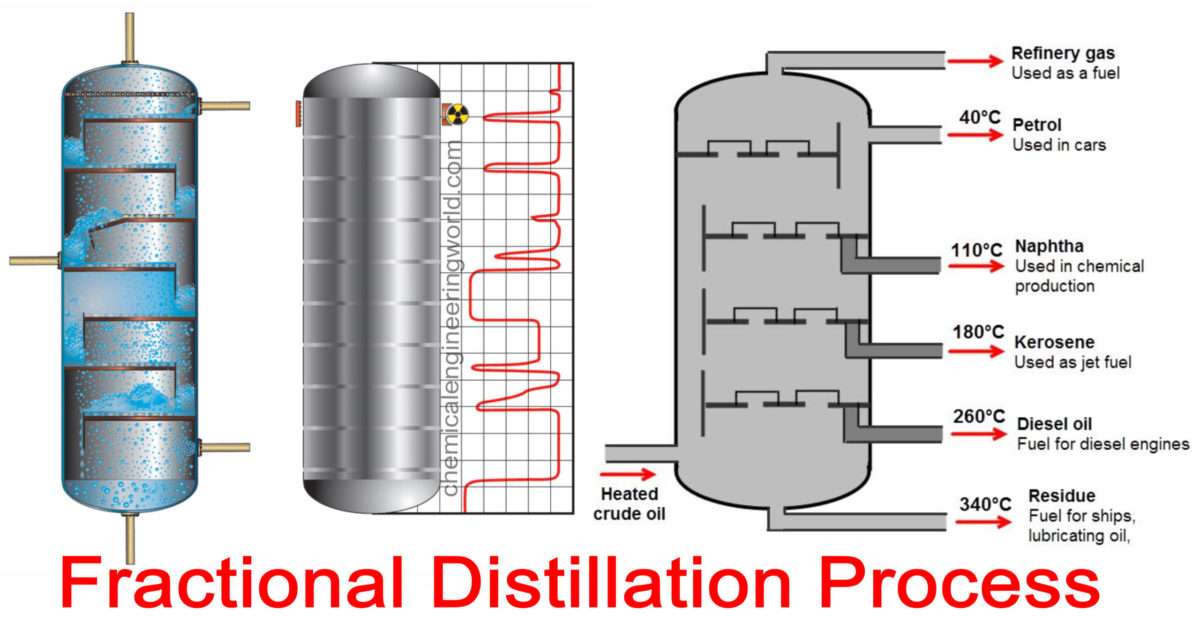
Fractional distillation - Energy Education Figure 1. Diagram of a fractional distillation tower, showing where the different fractions will condense.[1] Note that the temperature is higher at the bottom, so the longer carbon chains will fall out at the bottom, the shorter carbon chains will go up the column until they hit a temperature at which...
Atmospheric and Vacuum Distillation Units | FSC 432 ... A schematic diagram of atmospheric distillation unit illustrating the feed heat exchangers, pump around loops, and side steam strippers (adapted from [3]). Source: Dr. Semih Eser As shown in Figure 4.4, below (and in Figure 4.10 in the textbook), the atmospheric residue is reheated in a fired furnace to 730-850° F before introduction into the ...
Chem 211 - Techniques | Fractional Distillation Fractional distillation is a technique used when separating a mixture of two liquids that do not "behave" well enough to use simple distillation. Once you have set up your fractional distillation apparatus, place the liquid to be distilled in the distilling flask.
PDF Microsoft PowerPoint - Lecture 7 phase equilibrium phase of a substance. A phase diagram shows exactly what phases are present. at any given temperature and pressure. There are more modern ways to separate mixtures, for example, chromatography, but fractional distillation still has industrial and some laboratory importance.
Fractional distillation of crude oil - Fractional distillation - GCSE... Fractional distillation. The majority of our fuels and plastics are derived from oil. The diagram below summarises the main fractions from crude oil and their uses, and the trends in properties. Note that the gases leave at the top of the column, the liquids condense in the middle and the solids stay at...
fractional distillation of ideal mixtures of liquids Using the phase diagram On the last page, we looked at how the phase diagram for an ideal mixture of two liquids was built up. I want to start by looking again at Fractional distillation in the lab The apparatus A typical lab fractional distillation would look like this: Some notes on the apparatus The...
› physical › phaseeqiafractional distillation of ideal mixtures of liquids Fractional distillation industrially There is no difference whatsoever in the theory involved. All that is different is what the fractionating column looks like. The diagram shows a simplified cross-section through a small part of a typical column. The column contains a number of trays that the liquid collects on as the vapour condenses.
An innovative hollow fiber vacuum membrane distillation ... Feb 20, 2022 · The instantaneous phase separation during membrane-forming process induced the formation of a curved skinner layer on fiber lumen side which can be clearly seen from Fig. 4(b). In addition, it can be seen from the enlarged photos of the fiber outer skin layer ( Fig. 4 (c)) that a large number of sponge-like pores emerged in the outer skin layer ...
› qflab › 2020_21LABORATORY SESSION 6 Phase diagram: Boiling temperature ... temperature. These diagrams are needed when the aim is to separate the two liquids by fractional distillation. Figure 1 shows the phase diagram for an ideal solution. In a distillation experiment with constant pressure, the solution is heated and steam is extracted and condensed. The condensed liquid is richer in the more volatile component than in the original liquid. Fractional distillation repeats the boiling and condensation cycle several












0 Response to "43 fractional distillation phase diagram"
Post a Comment