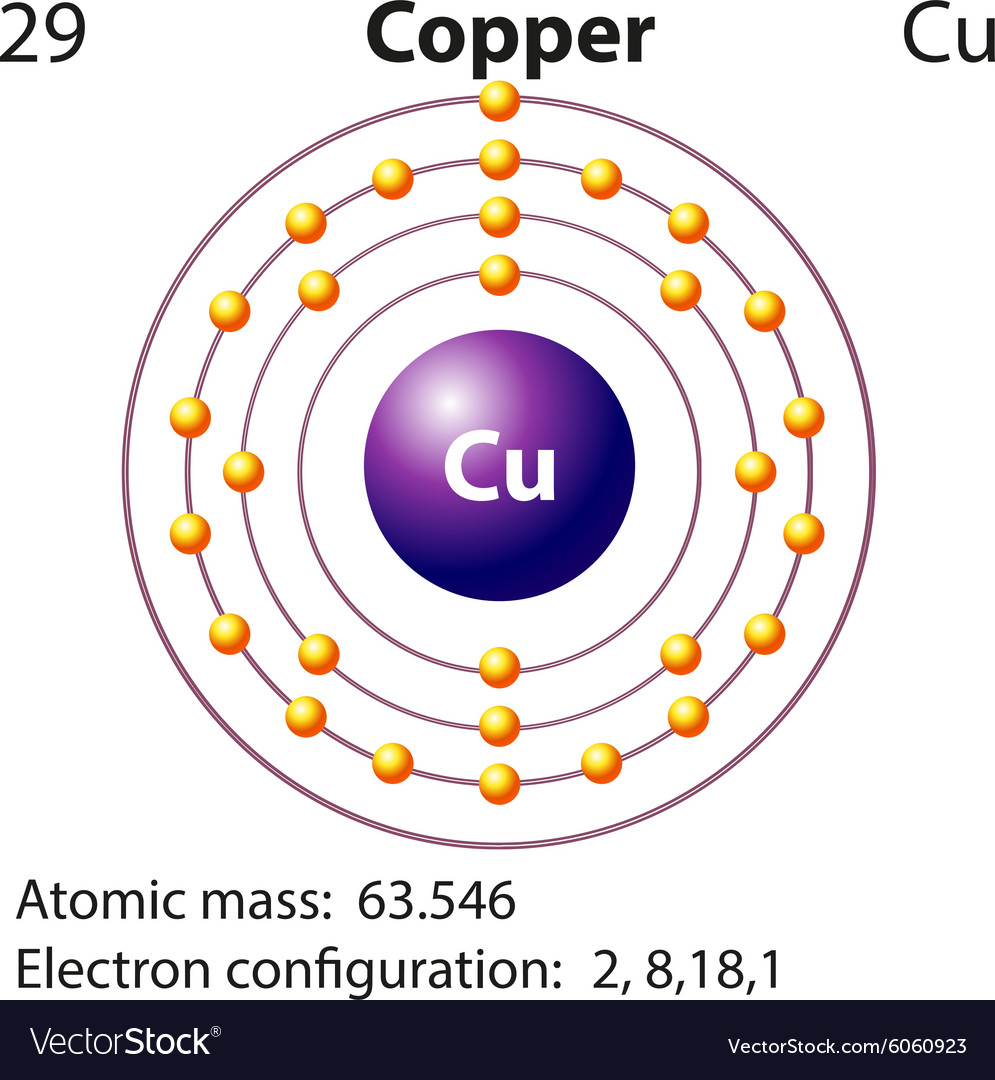
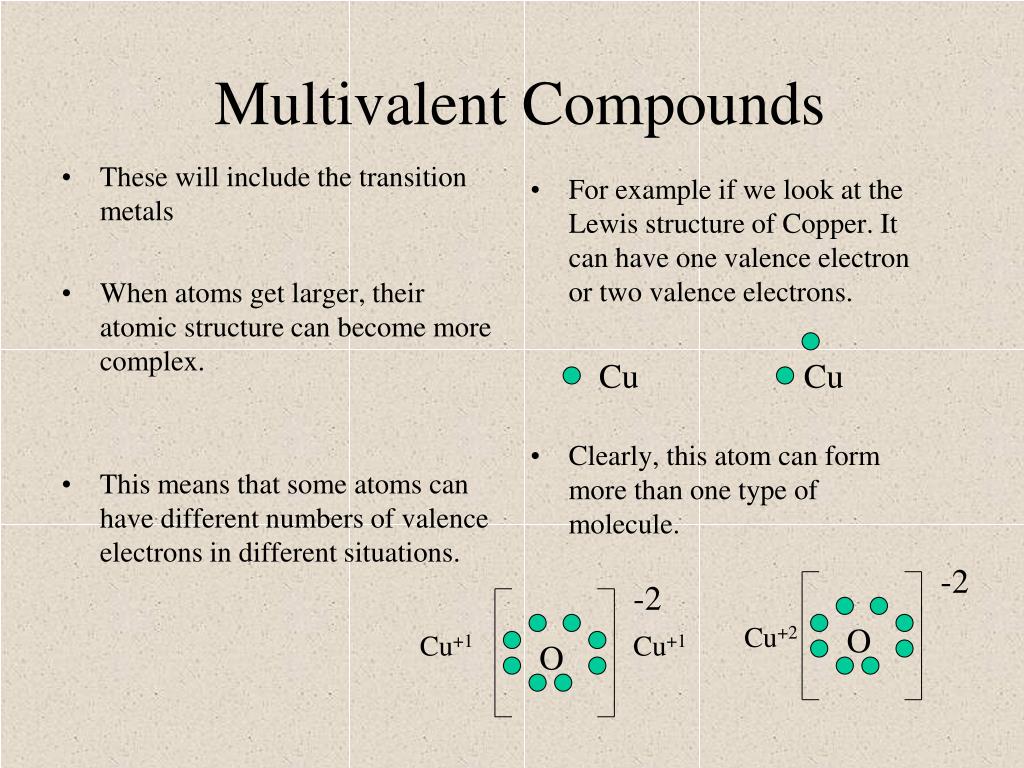
41 copper electron dot diagram
Carbon and Its Compounds Extra Questions - Class 10 ... Electron dot structure of ethane molecule, C 2 H 4. Que 7. ... Ethyl alcohol which contains a small amount of methyl alcohol or copper sulphate is called denatured alcohol. The purpose of denaturing the alcohol is to make it unfit for drinking purposes. Denatured alcohol is also used for industrial purposes. S2 Lewis Dot Structure - aunitedkingdomfilm.com Valence electrons are 8 2 in. S2 lewis dot structure. Around sulfur atom there are four bonds and a single lone pair in the lewis structure of SO 3 2-ion. SO2 Lewis structure sulfur dioxide electron dot structure is that type of diagram where we show the total 18 valence electrons of SO2 as dots or dots and dashes-In Lewis structureit is common ...
metallic bonding occurs between atoms of - best trick ... A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. The electrons are free to move throughout this electron sea. Metallic Bonding is a force that binds atoms in a metallic substance together. Answer 1 of 4. This forms a sea of electrons that surrounds the metal cations.
Copper electron dot diagram
Which statements describe kinetic and potential energy ... How do we draw a lewis dot diagram (electron dot diagram) for sodium ethyl xanthate? Please I'll mark brainliest for the correct answer. ... Heat will flow from the water in the glass to the copper until both reach the same temperature. C) The final temperature of the water in the glass and the copper will be 10 °C. ... Valence Electrons Chart for All Elements (Full Chart Inside) Valence electrons in Nihonium (Nh) 3. 114. Valence electrons in Flerovium (Fl) 4. 115. Valence electrons in Moscovium (Mc) 5. 116. › science › articleBioadhesive injectable hydrogel with phenolic carbon quantum ... Jan 01, 2022 · Electron spin resonance (ESR) spectroscopy analysis. ESR analysis was performed on an ESR spectrometer at 9.873 GHz (JES-FA200 ESR spectrometer, Japan). First, 2 mL of [email protected] solution (1 mg mL −1) was mixed with 1 mL APS solution (0.05 g mL −1) or 1 mL 30% H 2 O 2.
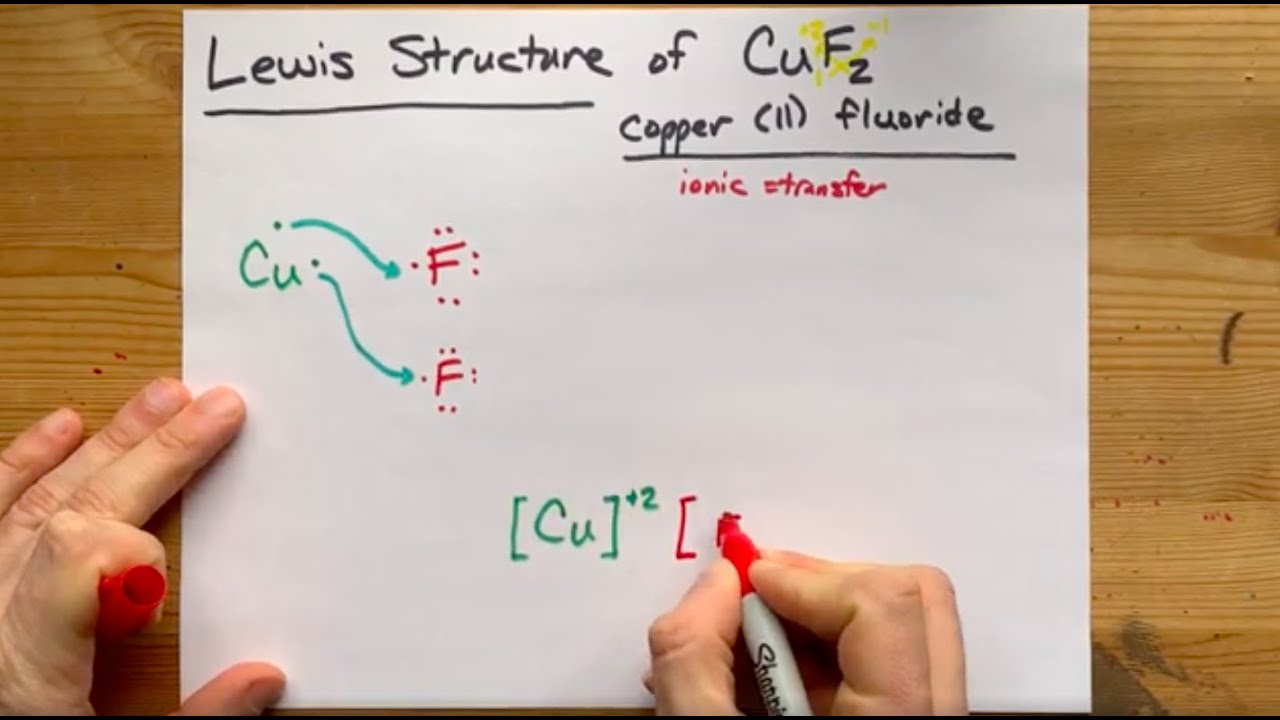
Copper electron dot diagram. how many chlorine atoms are there in 12.2 g of ccl4 ... A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. … Thus, a carbon atom will share each of its 4 outer electrons with a single chlorine atom, giving the single carbon atoms and 4 chlorine atoms a full outer shell of electrons.. How many carbon atoms are there in 4 molecules of CCl4? qualifications.pearson.com › content › damMark Scheme (Results - Edexcel Aug 22, 2018 · electron(s) and S gains electron(s) No M1 or M2 if mention of electron sharing or covalent bonding ALLOW Mg (ion) has a charge of 2+/+2 and S (ion) has a charge of 2-/-2 Two correct ionic half equations scores all 3 marks 3 Diagrams showing electron transfer and charges on the ions scores all 3 marks Electron transfer reactions question | Chemistry Help Forum Dec 22, 2021. #2. a)What happens if you mix each chemical. Stable is only Copper cathode/ Copperchloride sol./ Platinum (copper) anode. b) technical carried out, think about concentration of CuCl2. c) Not working. Reaction between Cu2+ and I- to unsolouble CuI. d)Cu/ CuCl2/ salt bridge/ Iodine/iodide/platinum. Last edited: Dec 22, 2021. Atomic Mass Gizmo Answer Key - Student Exploration Average ... Atom, atomic number, electron, electron dot diagram, element, energy level, ion, isotope, mass . All helium atoms have 2 protons. Consider the following equation for a chemical reaction: Answer to solved explorelearning date: Element builder gizmo™ shows an atom with a single proton. Calculate the molar mass of simple compounds with the aid ...
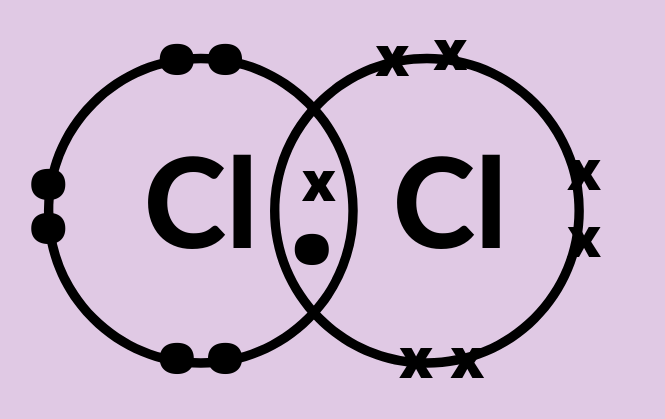
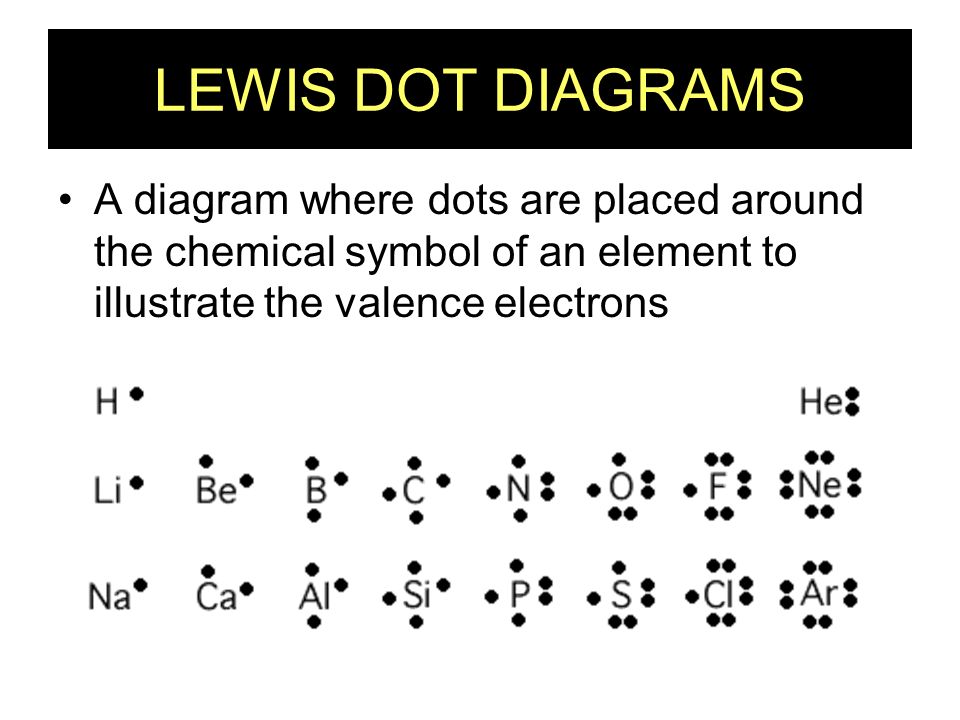
Chapter 8 - Chemical Bonds - CHE 105/110 - Introduction to ... A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. ... cobalt, nickel, copper, zinc, molybdenum, selenium, and iodine ... Metallic Bonding: The Electron-Sea Model & Why Metals Are ... Metallic bonding is known as the electron-sea model. Learn about metallic bonding with an explanation of the unique properties of metals, and understand why metals are good electrical conductors. S2 Lewis Structure 6 1 Lewis Electron Dot Diagrams Introductory Chemistry . Solved Question 25 Give The Number Of Valence Electrons For Chegg Com . 3 7 Lewis Structures Chemistry Libretexts . How To Determine The Lewis Dot Structure Of Copper Ii Sulfide Quora . Lewis Electron Dot Diagrams . Which Lewis Electron Dot Diagram Is Correct For A S 2 Ion Socratic › class › estaticsPhysics Tutorial: Conductors and Insulators 1. One of these isolated charged spheres is copper and the other is rubber. The diagram below depicts the distribution of excess negative charge over the surface of two spheres. Label which is which and support your answer with an explanation.
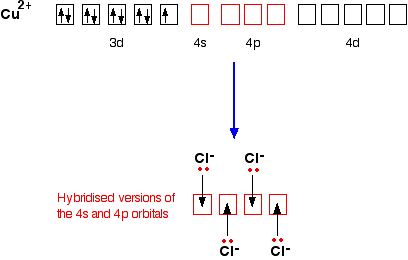
opentextbc.ca › introductorychemistry › chapterAtomic Theory – Introductory Chemistry – 1st Canadian Edition Which is larger, a neutron or an electron? What are the charges for each of the three subatomic particles? Where is most of the mass of an atom located? Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus. Sketch a diagram of a helium atom, which has two protons and two neutrons in its nucleus. Define atomic ... Explained: How Many Valence Electrons Does Calcium Have? Hydrogen, Sodium, and Lithium have only one valence electron in the group. Likewise, Neon, Krypton, and Argon have eight valence electrons in group 8. Electricity and Valence Electrons. The valence electrons determine the electricity of the atoms of an element. Moreover, the copper that is coated with plastic is a good electricity conductor. Atoms Elements And Compounds Ppt Tes - PowerPoint Presentation Covalent bonding. The atoms form a covalent bond by sharing their valence electrons to get a stable octet of electrons.(filled valence shell of 8 electrons). There are two electrons per bond, each atom donates one electron to the bond. Electron-Dot Diagrams of the atoms are combined to show the covalent bonds Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. ... Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: ... Electron configuration of elements Hund's rule and Orbital filling diagrams.
en.wikipedia.org › wiki › Spin_(physics)Spin (physics) - Wikipedia History. Wolfgang Pauli in 1924 was the first to propose a doubling of the number of available electron states due to a two-valued non-classical "hidden rotation". In 1925, George Uhlenbeck and Samuel Goudsmit at Leiden University suggested the simple physical interpretation of a particle spinning around its own axis, in the spirit of the old quantum theory of Bohr and Sommerfeld.
› embedWelcome to CK-12 Foundation | CK-12 Foundation FlexBook Platform®, FlexBook®, FlexLet® and FlexCard™ are registered trademarks of CK-12 Foundation.
Copper: Facts about the reddish metal that has been used ... Copper: Facts about the reddish metal that has been used by humans for 8,000 years. By Stephanie Pappas published 7 days ago. Copper is the only metal, aside from gold, whose coloring isn't ...
Activity B Average Atomic Mass Gizmo Answer Key ... Isotope information is provided below. Lithium-6 is 4 abundant and lithium-7 is 96 abundant. Atom atomic number electron electron dot diagram element energy level ion isotope mass number neutron nucleus periodic table proton radioactive valence electrons prior knowledge questions do these before using the gizmo note. The atomic mass of boron is ...
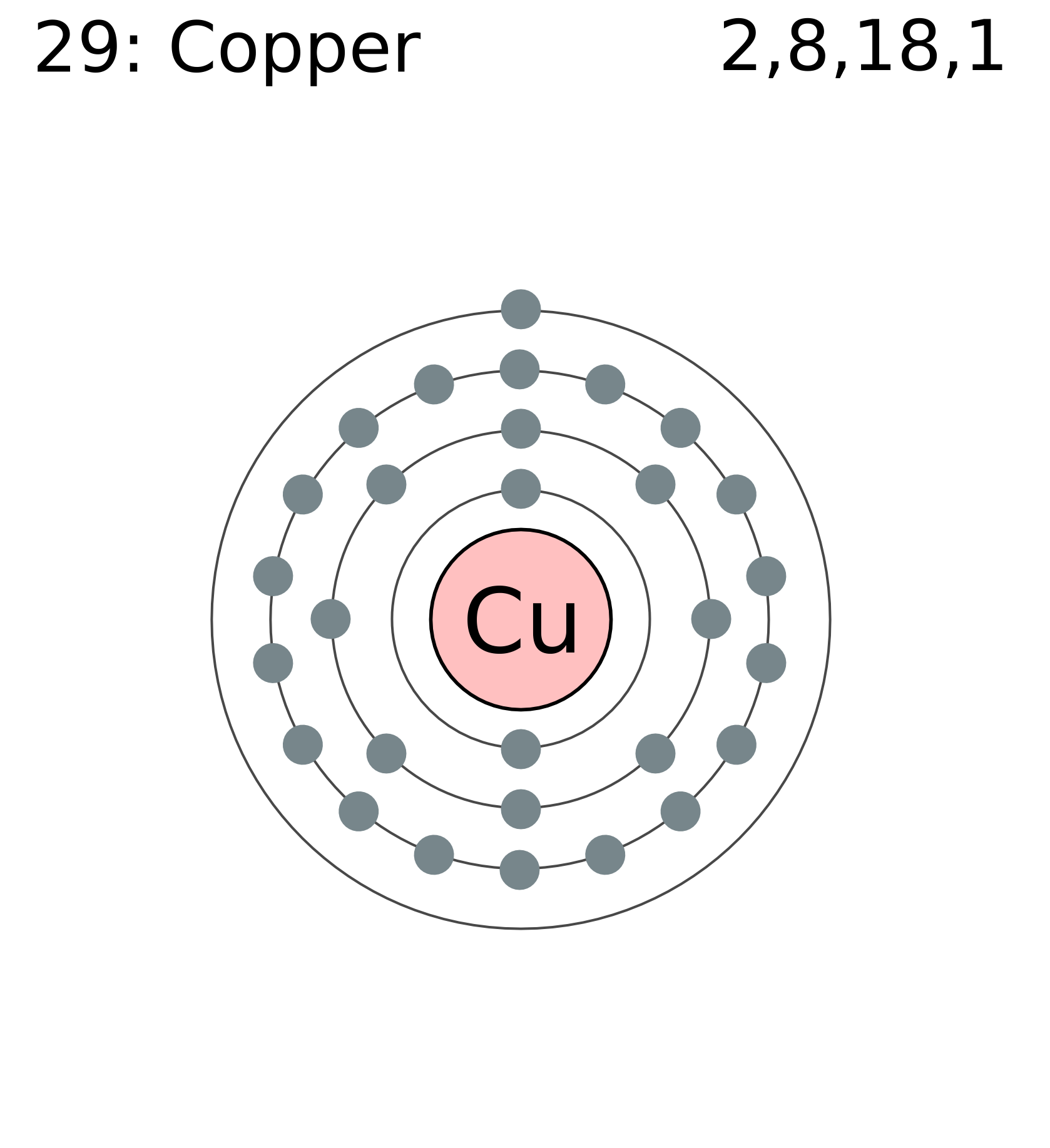
Orbital Copper Diagram [O1GH6Q] Copper (Cu) has an atomic mass of Find out about its Orbital Diagram. Since we began in 1998, copper and brass prices have quadrupled, or more. One way to extract the metal was to roast the sulfide ore then leach out the copper sulfate that was formed, with water. Then draw its electron dot diagram. .
what element is represented by the diagram - Lisbdnet.com Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below.
Lewis Structures: Learn How to Draw Lewis Structures ... This type of Lewis dot structure is represented by an atomic symbol and a series of dots. See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding. Example 1. Draw the Lewis Dot Structure for the Hydrogen atom. Since Hydrogen is in Group I it has one (1) valence electron in its shell. Example 2.
Boron Electron Dot Diagram - xxjasela #Boron Electron Dot Diagram How To Draw Lewis; Its atomic mass is 10.81 grams per mole. The physical properties of the molecule (like boiling point, surface tension, etc.).Boron is an element in the periodic table with a symbol B 5 is its atomic number.
Seznamy 32+ Argon Atom Diagram Electron dot diagram of an argon atom. Download scientific diagram | simplified energy level diagram of an argon atom. Visit chemical elements, crystals, melting points,bohr model of copper. As, from the bohr diagram of argon, we got to know, it has 8 valence electrons.
CBSE Class 10 Science Question Paper 2019 with Solutions (a) Draw electron dot structure of methane molecule. (b) Identify the functional groups present in the following compounds : (i) (ii) (c) A mixture of oxygen and ethyne is burnt for welding. Why do you think a mixture of ethyne and air is not used for welding ? View Solutions
cisce.org › UploadedFiles › PDFSCIENCE (52) Electron dot structure. (a) Electrovalent bonding: • Electron dot structure of Electrovalent compounds NaCl, MgCl. 2, CaO. • Characteristic properties of electrovalent compounds state of – existence, melting and boiling points, conductivity (heat and electricity), dissociation in solution and in molten state to be linked with electrolysis.
Electron dot diagrams are a way to illustrate the number ... Electron dot diagrams are a way to illustrate the number of valence electrons for an element. The symbol for the element is surrounded by a dot for each valence electron. Match the following electron dot diagrams with an element: 1. 2. 3. 4.
Revision Notes for Atomic Structure and Chemical Bonding ... In the reaction, hydrogen acts as a reducing agent and reduces copper oxide to copper. This is a reduction reaction. Reduction: Cu +2 + 2e-→ Cu. Simultaneously, copper oxide acts as a oxidizing agent and oxides hydrogen to water and this is an oxidation reaction. ... Electron dot Structural Diagram. Atomic or Orbit Structural Diagram ...
Lakhmir Singh Class 10 Chemistry 4th Chapter Carbon And ... Write down (i) structural formula, and (ii) electron-dot formula, of any one isomer of hexane (C6H14), other than n-hexane. 21) Fill in the following blanks with suitable words : (a) Ans: The form of carbon which is known as black lead is graphite .
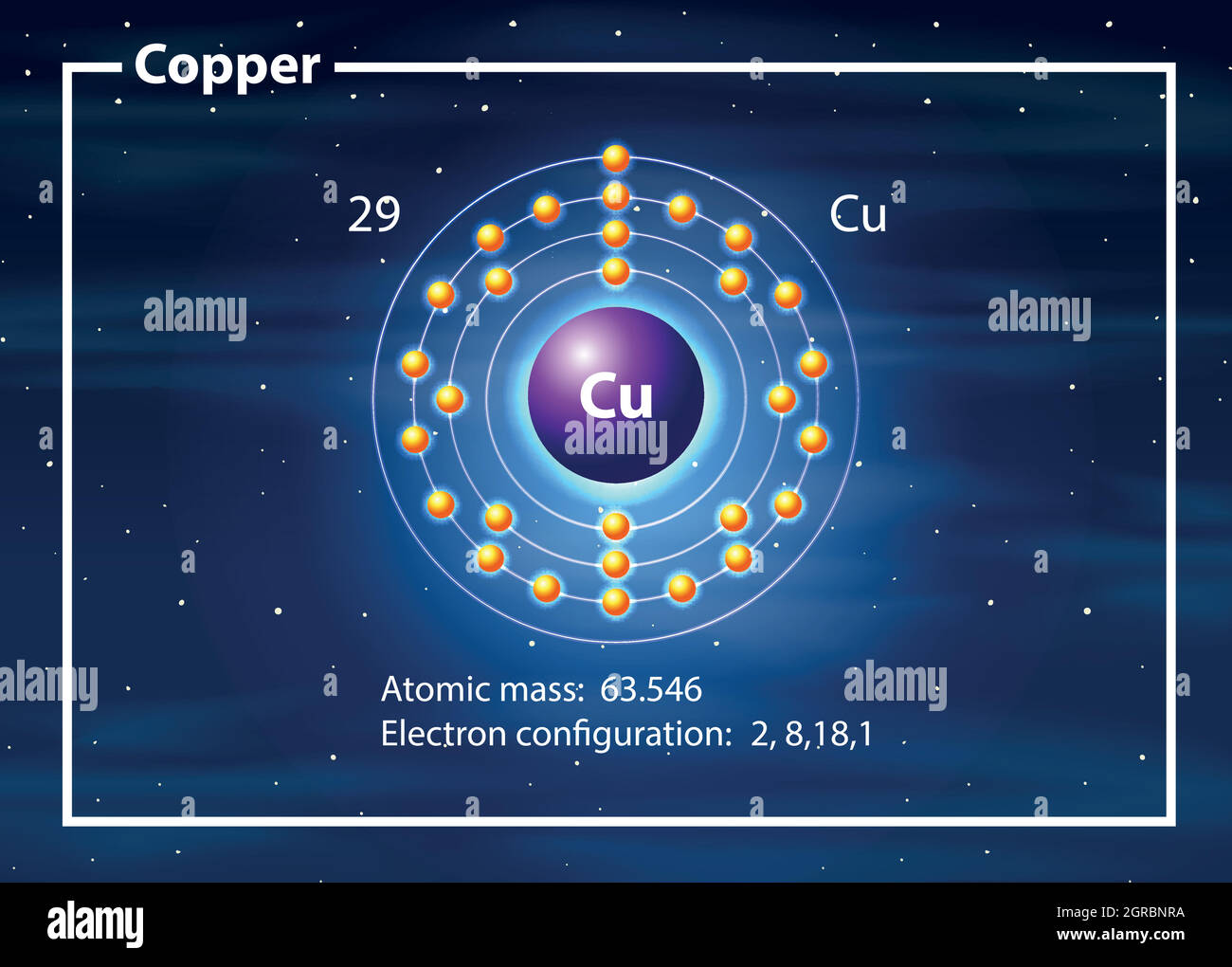
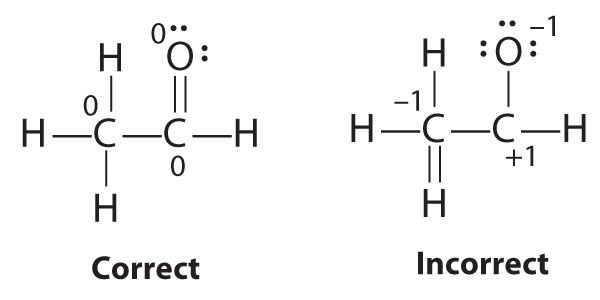
Why is it difficult to draw an atom of copper? - Brainly.com In order to write the Copper electron configuration we first need to know the number of electrons for the Cu atom (there are 29 electrons). Once we have the configuration for Cu, the ions are simple. ... Explain what is wrong with the following Lewis Dot diagram? if the mixture is 130.0 grams what is the mass of the water 1. 30.0 g 2. 70.0 g 3 ...
Enhancing the mechanical-electrical property ... In the development of copper-based composite materials, the dilemma of improving the mechanical properties without affecting the electrical properties is an important issue that must be solved. Here, carbonized polymer dot (CPD), as a novel reinforcement, was employed to fabricate CPD/Cu (pure copper) composite via powder metallurgy technique for the first time. The microstructure analysis ...
02/16 Super Tic Tac Toe Write the unabbreviated electron configurations of the following elements:a) copperb) iodine, Write the abbreviated electron orbital diagram for the following element:Krypton, Write the abbreviated electron configurations of the following elements:a) iridiumb) chlorine
How many grams would 3.36 × 1023 molecules of copper (ii ... Draw the electron-dot structure for chclo. draw the molecule by placing the atoms on the grid and connecting them with bonds. include; How much energy is required to heat 36.0 g H2O from a liquid at 65°C to a gas at 115°C? How many moles of carbon (c) atoms are equivalent to 38.1 g c?
› science › articleBioadhesive injectable hydrogel with phenolic carbon quantum ... Jan 01, 2022 · Electron spin resonance (ESR) spectroscopy analysis. ESR analysis was performed on an ESR spectrometer at 9.873 GHz (JES-FA200 ESR spectrometer, Japan). First, 2 mL of [email protected] solution (1 mg mL −1) was mixed with 1 mL APS solution (0.05 g mL −1) or 1 mL 30% H 2 O 2.
Valence Electrons Chart for All Elements (Full Chart Inside) Valence electrons in Nihonium (Nh) 3. 114. Valence electrons in Flerovium (Fl) 4. 115. Valence electrons in Moscovium (Mc) 5. 116.
Which statements describe kinetic and potential energy ... How do we draw a lewis dot diagram (electron dot diagram) for sodium ethyl xanthate? Please I'll mark brainliest for the correct answer. ... Heat will flow from the water in the glass to the copper until both reach the same temperature. C) The final temperature of the water in the glass and the copper will be 10 °C. ...
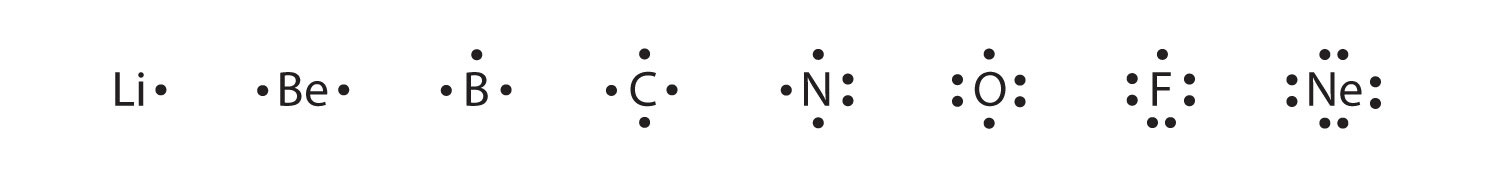




:max_bytes(150000):strip_icc()/Lead-58b601095f9b5860464ba934.jpg)




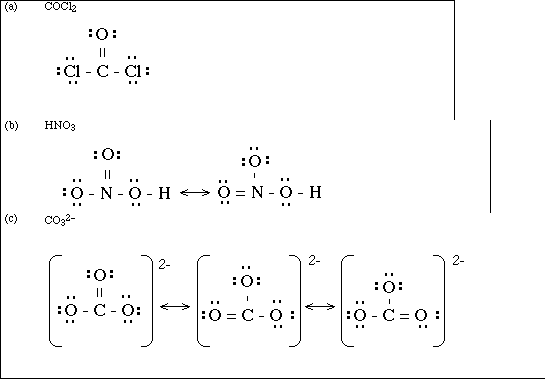
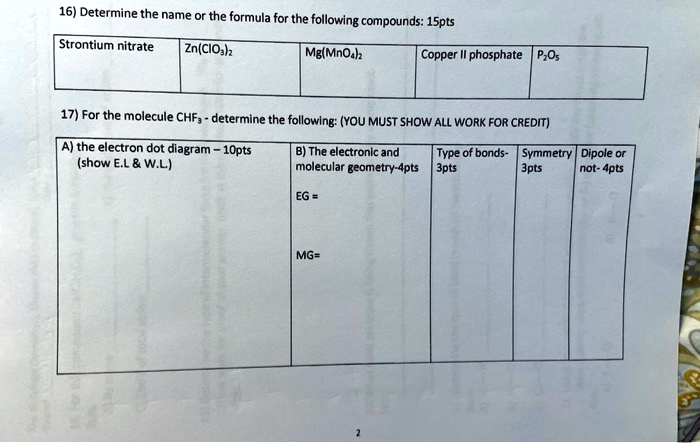







0 Response to "41 copper electron dot diagram"
Post a Comment