42 b2 2+ molecular orbital diagram
What Is The Bond Order Of B2 1 So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond. Nov 11, 2016 What is the molecular orbital diagram for C_2^-? | Socratic Explanation: The problem provides you with the MO diagram for the C2 molecule, so all you really have to do here is add an electron to that diagram. You need to add an electron and not remove one because of the overall negative charge that exists on the molecule. As you know, a neutral carbon atom has a total of 6 electrons.
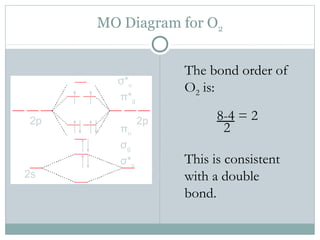
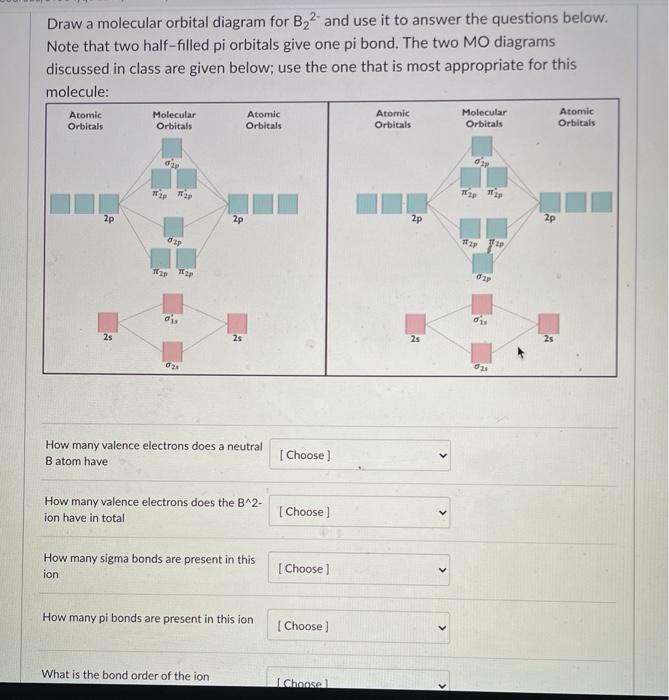
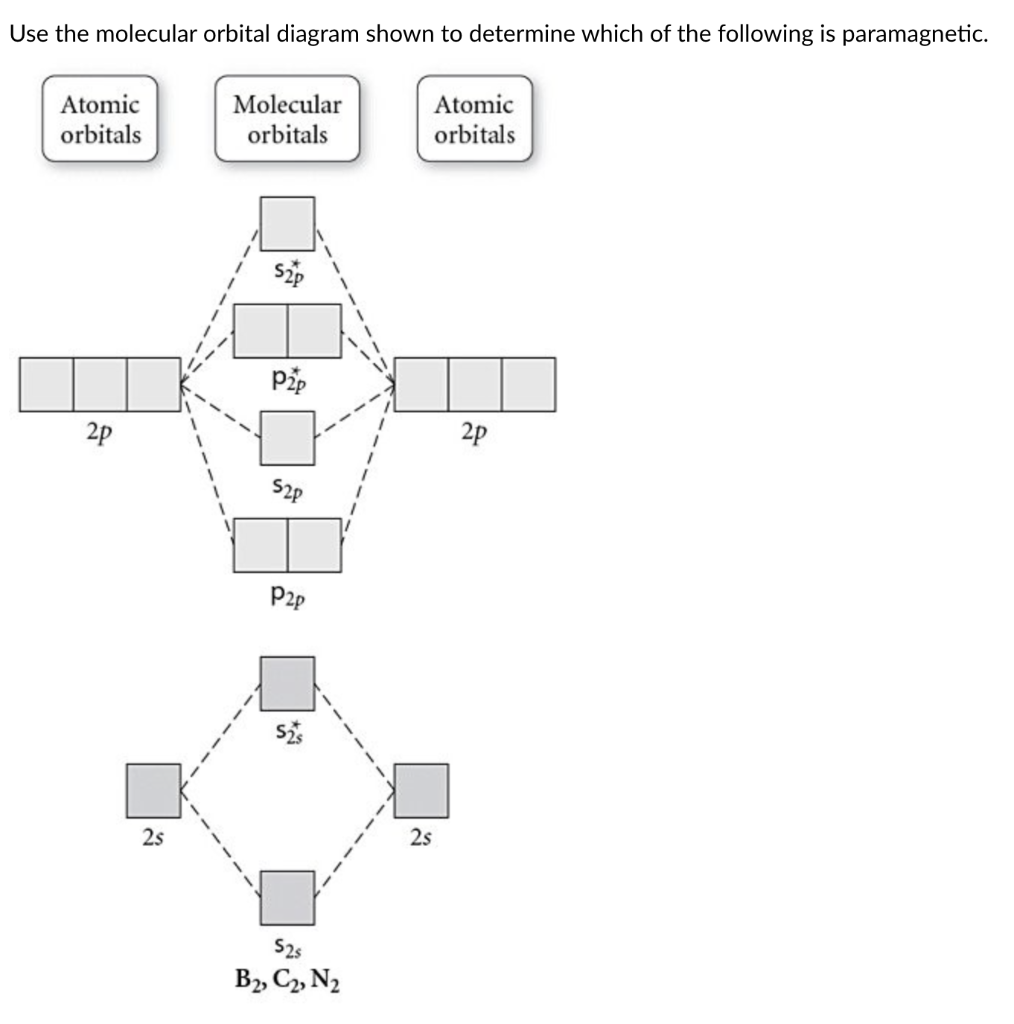
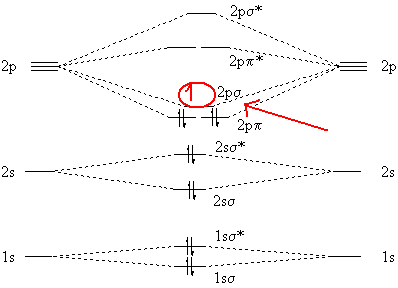
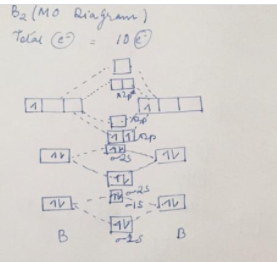
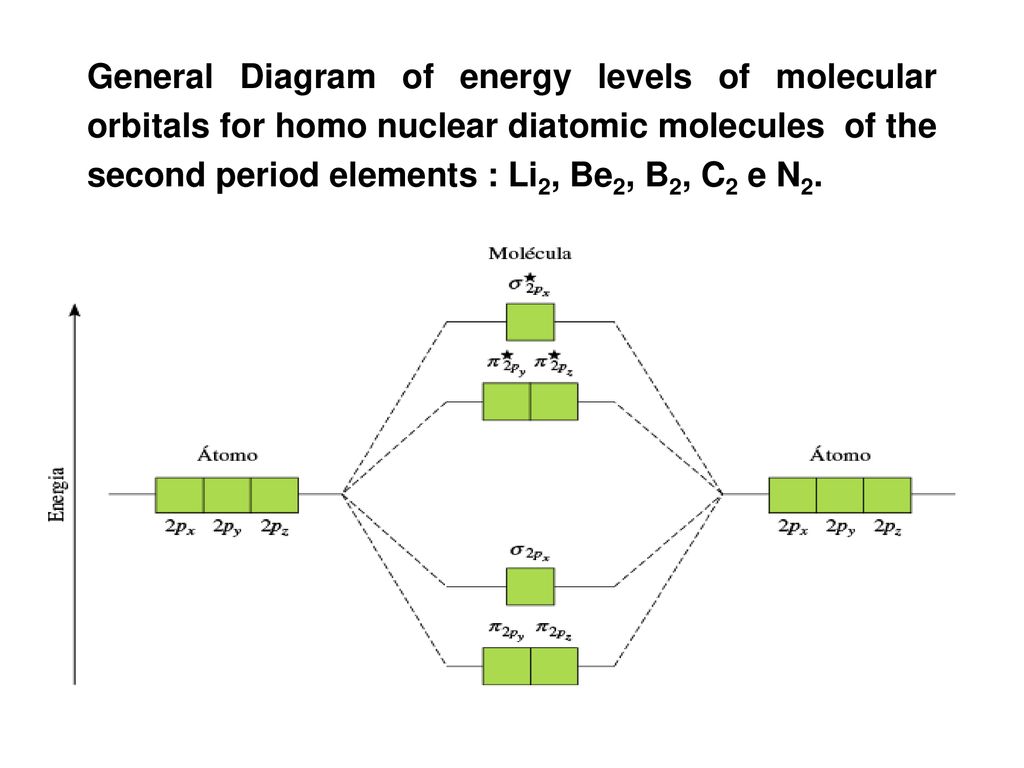
Be2 Molecular Orbital Diagram - schematron.org In general, bonding molecular orbitals are lower in energy than either of their parent atomic orbitals. Nov 11, · As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond.
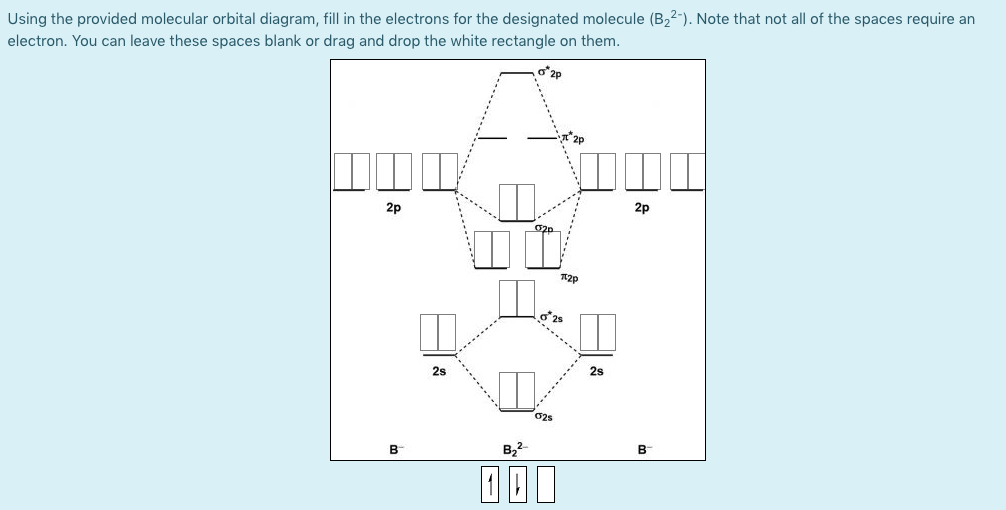
B2 2+ molecular orbital diagram
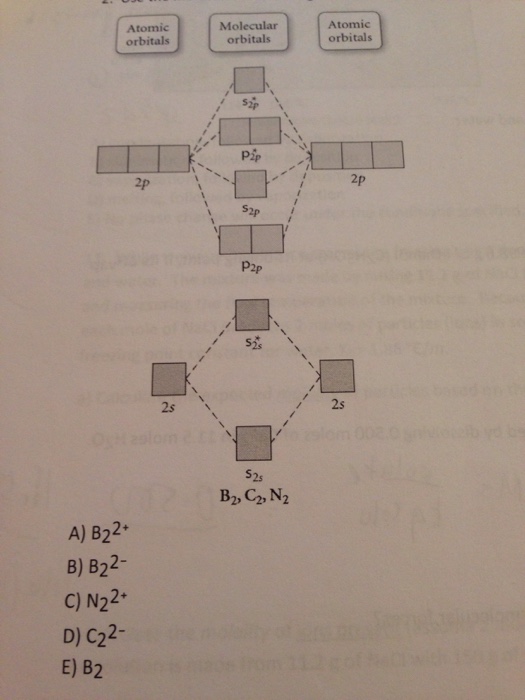
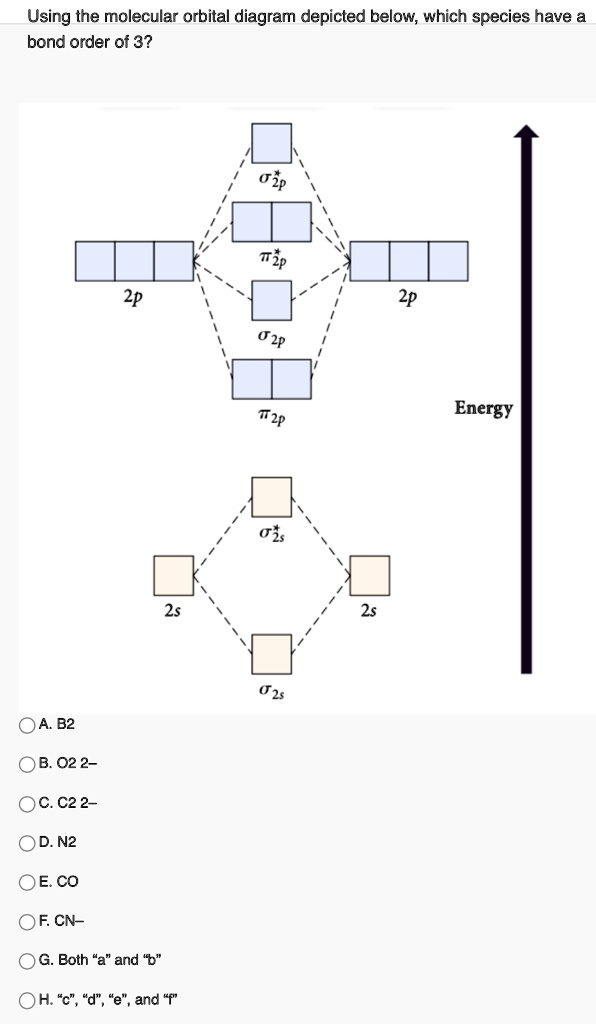
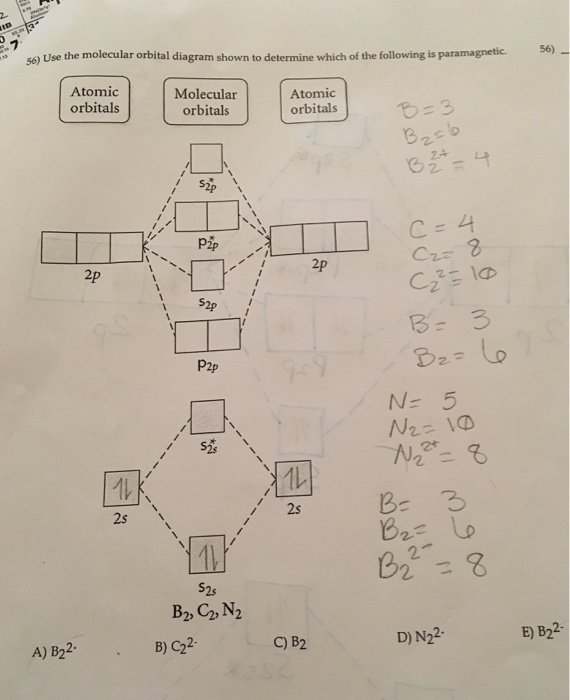
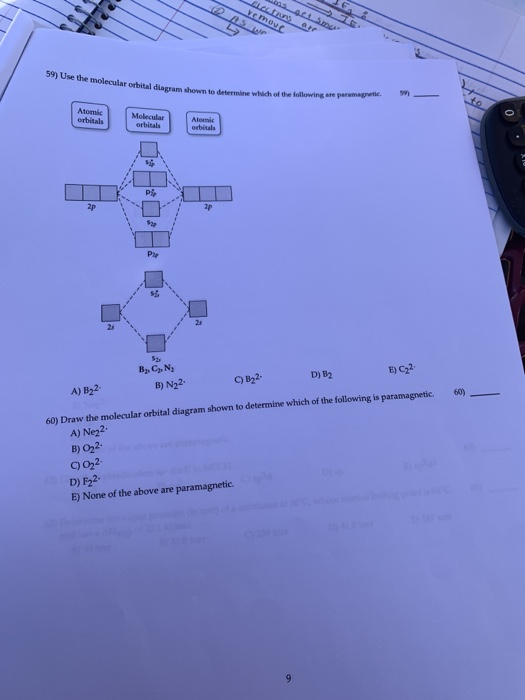
Recitation Week 10 (test 3 - Recitation 2) - GitHub Pages 2) Use molecular orbital diagrams to determine which of the following are paramagnetic. A) O2^2- B) Ne2^2+ C) O2^2+ D) F2^2+ E) None of the above are paramagnetic 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2- C) N2^2+ D) C2^2- E) B2 Solved Molecular Orbital theory predicts that the B2 2 ... Molecular Orbital theory predicts that the B2 2- ion would have a bond order of _____. If one of the highest valence electron(s) was excited into the next highest energy molecular orbital, it would_____. a. 1,strengthen the bonding in the molecule b. 2, have no effect on the bonding strength of the molecule c. 2, strengthen the bonding in the ... M.O. Diagram for B2 - CHEMISTRY COMMUNITY When you write the M.O. Diagram for B2, this is what you get: This shows two unpaired electrons in π2px and π2pz. However, the Bond Order of B2 = 1/2 (4-2) = 1. Doesn't this mean that B2 has a single bond between the two Boron atoms--therefore they have one sigma bond?
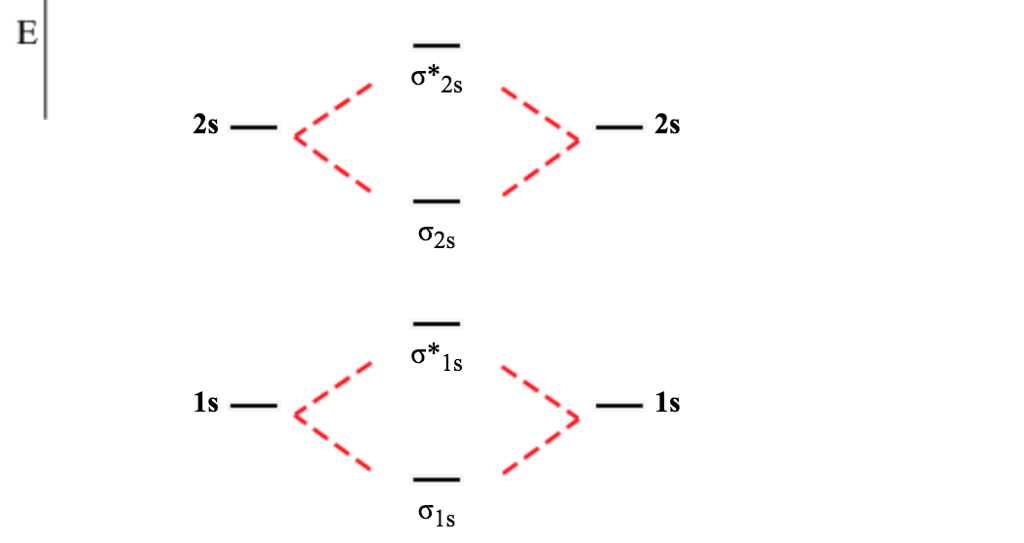
B2 2+ molecular orbital diagram. Bh2 Molecular Orbital Diagram 2 linear. BH2. 2a1. 21b2. 23a1. MO Diagram for BeH2. Be 2p. Be 2s. -. +. BeH2 MOs bonding MOs antibonding MOs non-bonding orbitals. Be AOs. H LCAOs g u u bonding nonrbonding. a complex MO diagram: B2H6 MO diagrams combine two fragments. Symmetry .. fragment =5e therefor keep up to b2 orbital z x y b1 a1 a1 b2. BH2. Fig. Orion Constellation (the Hunter): Stars, Facts, Myth ... Pi-2 Orionis (2 Orionis) is also a main sequence dwarf, belonging to the spectral class A1Vn. It is located 194 light years from Earth and has a visual magnitude of 4.35. Pi-3 Orionis (1 Orionis), also known as Tabit, is the brightest of the six stars. It is a white dwarf belonging to the spectral class F6V, only 26.32 light years distant from ... Molecular Orbital Theory. B2 - YouTube This video shows the end of the Be2 molecule MO diagram and explains pi orbitals, paramagnetism, and the MO diagrams for B2. Li2- Molecular Orbital Diagram Molecular orbital diagram of H 2 (Hydrogen molecule). As a simple MO example, consider the electrons in a hydrogen molecule, H 2 (see molecular orbital diagram), with the two atoms labelled H' and H". The lowest-energy atomic orbitals, 1s' and 1s", do not transform according to the symmetries of the molecule.
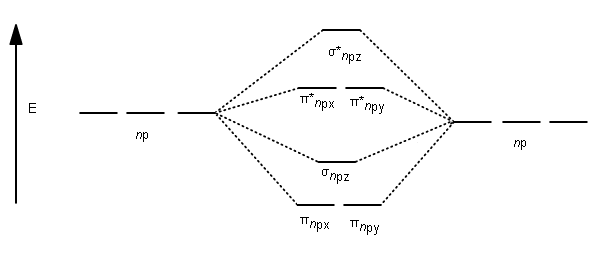
Solved Draw the molecular orbital diagram for B2+ (this is ... Science Chemistry Chemistry questions and answers Draw the molecular orbital diagram for B2+ (this is not B-B. it has an electron missing, so its B-B cation!) The number of unpaired electrons in the B2+ molecule is 2 (two) 1 (one) 3 (three) zero Quiz 1 Flashcards | Quizlet 2. The bond order of a homonuclear diatomic molecule can be decreased by. removing electrons from a bonding MO or adding electrons to an antibonding MO. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) N2^2+. B) B2^2+. C) B2^2-. D) C2^2-. E) B2. Why is there a difference between O2 and B2 sigma 2p ... The σ 2 s MO will obtain somewhat p character. So in a energy level MO diagram σ 2 p bonding orbital is placed above the π 2 p orbital. However, in case of O X 2, due to the higher nuclear charge of oxygen, the energy difference of 2 s and 2 p is large so there is no such kind of intermixing between the bonded MOs. Draw the molecular orbital diagram for:(i) Be2(ii) B2 and ... The molecular orbital electronic configuration, Magnetic property: Since bond order is zero, Be 2 molecule does not exist. It is diamagnetic due to the absence of any unpaired electron. B 2 molecule: The electronic configuration of B atom (Z = 5) is. B 2 molecule is formed by the overlap of atomic orbitals of both boron atoms. A number of ...
Li2- Molecular Orbital Diagram - schematron.org Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = (). B.O = 1. Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation. Answer to Draw a molecular ... is b22+ paramagnetic or diamagnetic Since it posses 2 unpaired electrons, it is Paramagnetic in nature. A b22 b b22 c n22 d c22 e b2. We now turn to a molecular orbital description of the bonding in \(\ce{O2}\). IOCTL_STORAGE_PROTOCOL_COMMAND support Vendor cmd only. B f22 c ne22 d o22 e f22 2 use molecular orbital diagrams to determine which of the following are paramagnetic. O ... Which of the following diatomic species are paramagnetic ... A blank molecular orbital diagram (Part B 1 figure) has been provided to help you. Drag the formulas to the appropriate magnetic bin :C2^2+,Li2-,B2^2- Science. which of the following are predicted by the molecular orbital model to be stable diatomic species N22-, O22-, F22- Chemistry HELP ASAP Answered: Draw the molecular orbital diagram… | bartleby Consider these following ions: O2-, N22-, Li2+ and O22- a. Based on molecular orbital theory (MOT), which of the ion(s) exhibit(s) paramagnetism? b. For those ions that are paramagnetic, determine the number of unpaired electrons. Support your answers with draw appropriate molecular orbital energy diagram.
Atomic Orbitals in Molecular Orbital Diagrams - CHEMISTRY ... Atomic Orbitals in Molecular Orbital Diagrams. Moderators: Chem_Mod, Chem_Admin. 2 posts • Page 1 of 1. Sohee Park 3E Posts: 11 Joined: Fri Sep 25, 2015 10:00 am. ... Image of B2 2+ is below. You do not have the required permissions to view the files attached to this post. Top ...
What is the molecular orbital diagram for B_2? | Socratic Jan 27, 2015 Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals.
What is the molecular orbital configuration of C2? 2022 2. What is the molecular orbital configuration of C2? Looking at the appropriate MO diagram, we see that the π orbitals are lower in energy than the σp orbital. The valence electron configuration for C2 is (σ2s)2(σ∗2s)2(π2py,π2pz)4 ( σ 2 s ) 2 ( σ 2 s ∗ ) 2 ( π 2 p y , π 2 p z ) 4 . [ad_2]
Schematic drawings of the HOMO and LUMO of compound B2 ... Download scientific diagram | Schematic drawings of the HOMO and LUMO of compound B2. from publication: QSAR Studies, Electronic Structure, Drug Likeness of 1,2-Dithiole-3-One Derivatives ...
Molecular Orbital (MO) Diagram for F2(2+) - YouTube When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so ...
Draw MOT diagram for B2 molecule and calculate its class ... The molecular orbitals having the same sign combine and give bonding molecular orbitals. We have to draw the molecular orbital diagram for B 2 molecule. The B 2 molecule is formed by the combination of two boron atoms. The two boron atoms are linked by a covalent bond. The atomic number of boron is 5.
M.O. Diagram for B2 - CHEMISTRY COMMUNITY When you write the M.O. Diagram for B2, this is what you get: This shows two unpaired electrons in π2px and π2pz. However, the Bond Order of B2 = 1/2 (4-2) = 1. Doesn't this mean that B2 has a single bond between the two Boron atoms--therefore they have one sigma bond?
Solved Molecular Orbital theory predicts that the B2 2 ... Molecular Orbital theory predicts that the B2 2- ion would have a bond order of _____. If one of the highest valence electron(s) was excited into the next highest energy molecular orbital, it would_____. a. 1,strengthen the bonding in the molecule b. 2, have no effect on the bonding strength of the molecule c. 2, strengthen the bonding in the ...
Recitation Week 10 (test 3 - Recitation 2) - GitHub Pages 2) Use molecular orbital diagrams to determine which of the following are paramagnetic. A) O2^2- B) Ne2^2+ C) O2^2+ D) F2^2+ E) None of the above are paramagnetic 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2- C) N2^2+ D) C2^2- E) B2













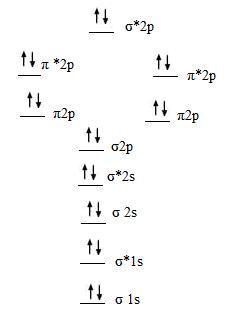






0 Response to "42 b2 2+ molecular orbital diagram"
Post a Comment