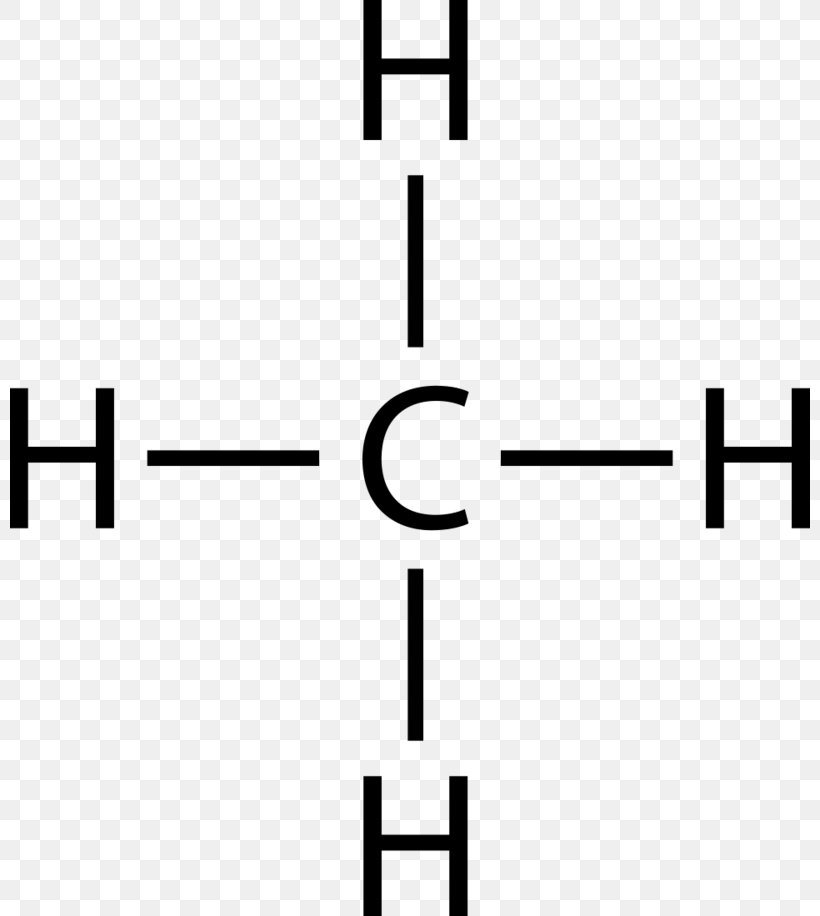
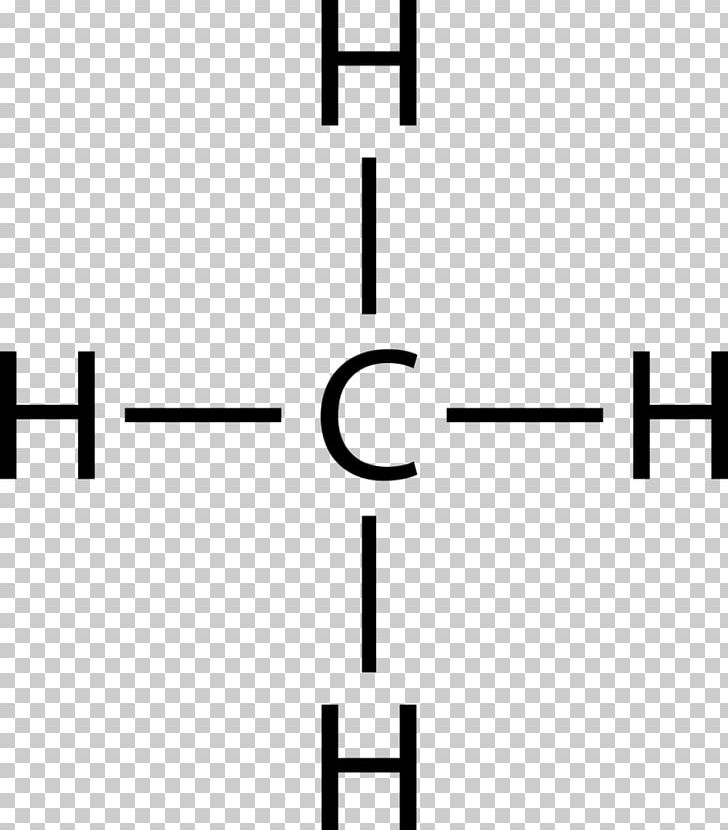
44 lewis diagram for methane
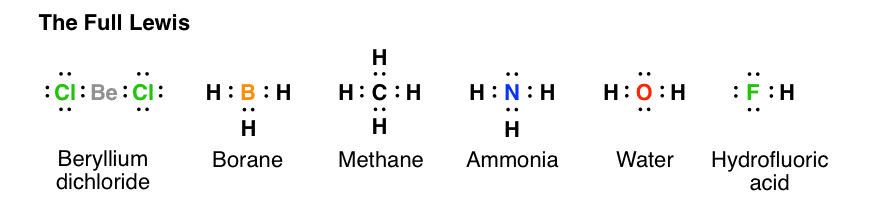
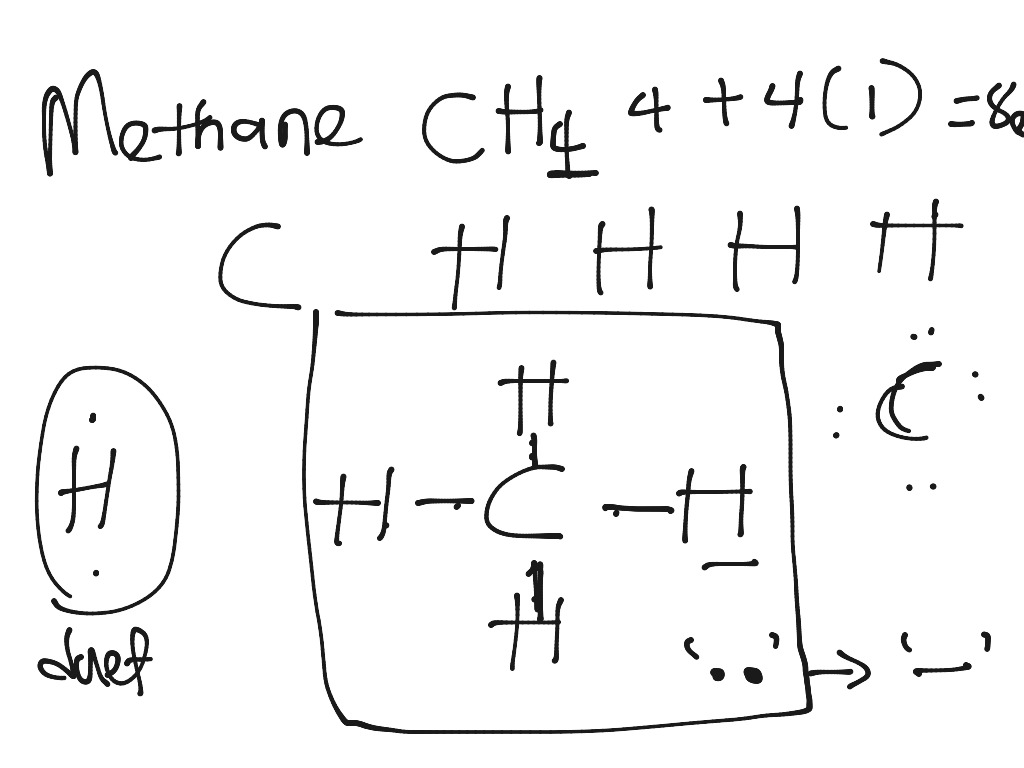
In the given molecule methane, CH₄: the valence configuration for C = 2s²2p² the valence configuration for H = 1s¹ Since two electrons are required to form a bond, the C atom in methane can form one bond with each of the 4 H atoms. Therefore, in the Lewis diagram, the C atom will be in the center surrounded by the 4 H atoms. Advertisement Survey
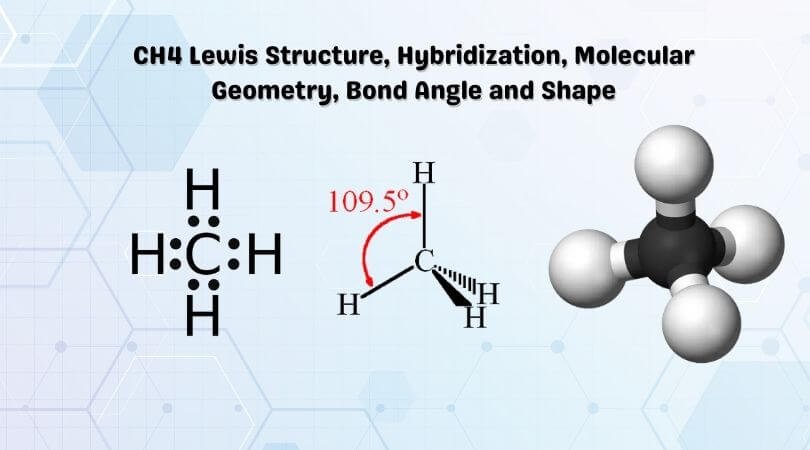
Methane (CH4) is a colourless, odourless, and highly combustible gas that is utilized to generate energy and heat houses all over the world.CH4 Lewis structure comprises two different atoms: Carbon and hydrogen. It is a nonpolar molecule with a bond angle of 109.5° degrees. CH4 is utilized in chemical processes to create other essential gases such as hydrogen and carbon monoxide, as well as ...
Lewis Structure Of Hydrogen Methane, Liaison covalente: définition et explications, methane Definition, Properties, Uses, & Facts Britannica, Organic Chemistry: Alkanes and Halogenated Hydrocarbons, File:Ammonia 2D svg Wikimedia Commons

Lewis diagram for methane
Methane (CH 4) Molecule Lewis Structure Methane lewis structure contains four C-H bonds. There are no lone pairs in the valence shells of carbon atom. Carbon atom is the center atom and it is very easy to draw CH4 lewis structure. CH 4 lewis structure There are following specifications in the lewis structure of methane.
Methane is a one-carbon compound in which the carbon is attached by single bonds to four hydrogen atoms.It is a colourless, odourless, non-toxic but flammable gas (b.p. -161℃). It has a role as a fossil fuel, a member of greenhouse gas and a bacterial metabolite.
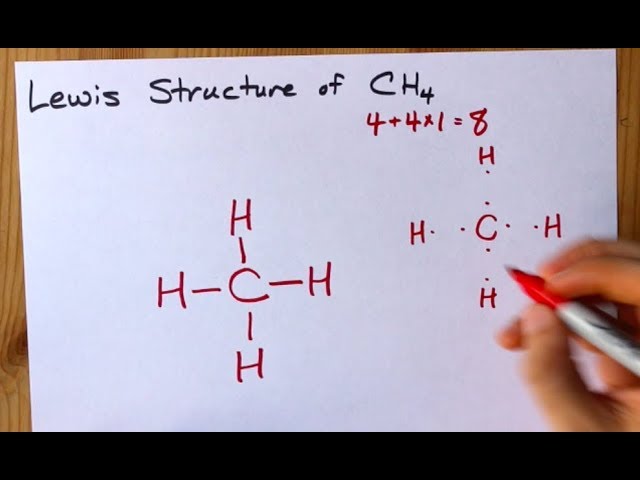
Drawing the Lewis Structure for CH 4. For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single bonds.It's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.
Lewis diagram for methane.
The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3.
18 May 2020 — The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots.
Draw A Lewis Dot Structure For Ch4, ch4 lewis dot diagram structure structures draw methane electron bonds each ch dots created shown, ch4 lewis dot diagram structure draw molecular estructura hcl methane cross, ch4 dot diagram lewis structure electron methan formel determine lg, dot methane structure electron diagram lewis ch4 diagrams bonding covalent draw bond chemistry hi chemical chem ...
Properties and bonding. Methane is a tetrahedral molecule with four equivalent C-H bonds.Its electronic structure is described by four bonding molecular orbitals (MOs) resulting from the overlap of the valence orbitals on C and H.The lowest-energy MO is the result of the overlap of the 2s orbital on carbon with the in-phase combination of the 1s orbitals on the four hydrogen atoms.
A Lewis Dot Structure is drawn by a series of dots, lines, and atomic symbols and provides a structure for the way that the atom or molecule is arranged. A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures
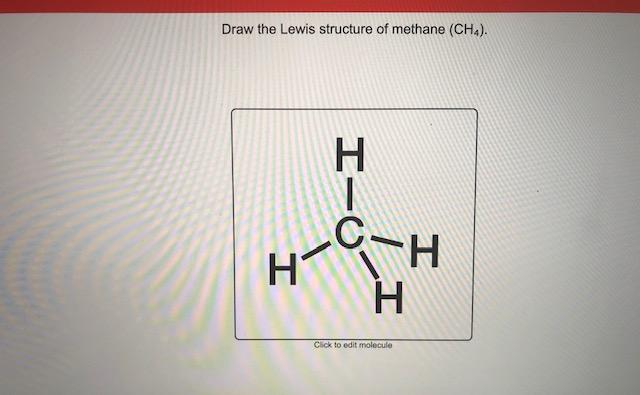
The steps for drawing the Lewis structure for the methane molecule is as follows: Figure 02: Lewis Structure of Methane Molecule. The chemical formula of methane is CH4. The carbon atom is less electronegative than the hydrogen atom. Therefore, the central atom of the molecule is carbon.
Dr. B. explains how to draw the Lewis dot structure for CH 4 (methane). The CH 4 Lewis Structure is one of the most frequently tested Lewis Structures.. Note that hydrogen atoms always go on the outside of a Lewis dot structure. This is because they can share a maximum of two electrons.
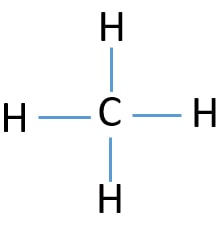
The Lewis structure of methane shows a central atom surrounded by four separate regions of high electron density. Each region consists of a pair of electrons bonding the carbon atom to a hydrogen atom. According to the VSEPR model, these regions of high electron density spread out from the central carbon atom in such a way that they are as far ...
CH4 Lewis structure (Methane electron dot structure) is that type of diagram where we show the total 8 valence electrons of CH4 as dots , or dots and dashes (-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash (-) or dots ( ) but a lone pair of two electrons is shown by dots [ ]. Explanation:
Drawing the Lewis structure for CH 4 (named methane) requires only single diagramweb.net's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. Lewis dot structure of CH 4.
How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use...
The Lewis structure for methane: a. shows that this is a binary compound. b. shows that C has 4 single covalent bonds. c. shows that there are 4 H's bonded to C in a regular tetrahedron 3D shape.
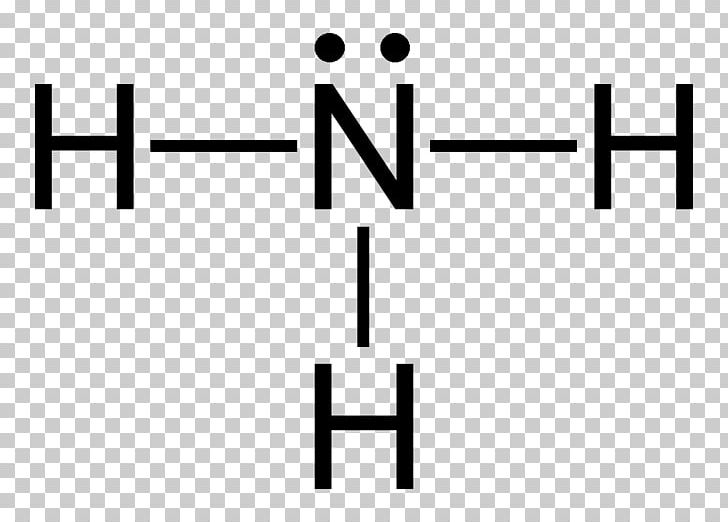
A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. When constructing a Lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share electrons until they are surrounded by eight ...
The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3.
Check me out: http://www.chemistnate.com
Lewis Dot Structures 1. Methane - CH 4 Number of Valence Electrons: 4 from C and 1 each from 4 H = 8 Carbon is more electronegative than hydrogen, but hydrogen can never be the "central" atom, as it can only form 1 bond. Carbon always forms 4 bonds (2 electrons each). 2. Ammonia - NH 3
CH4 Lewis Structure, Hybridization, Molecular Geometry, Bond Angle and Shape. Methane is one of the simple organic molecules, given its straightforward structure. It has the chemical formula of CH4 and comprises one carbon atom forming bonds with four hydrogen atoms. The compound is one of the main constituents of natural gas.
Hydrogen only has one valence electron and can only share one. Methane's (When it comes to hydrocarbons, “meth” stipulates one carbon, “ane” stipulates a single ...1 answer · 4 votes: Well Carbon only has 4 valence electron, so it can bond at all four point. Hydrogen only ...
Lewis Dot Structure for CH4 #2 Find the number of "octet" electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 . We show two ways to draw the CH4 Lewis structure, methane. We also have This info can then be used to determine the Lewis Dot Structure. Find an answer to your question draw the ...
The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3.
The Lewis dot structure for methane (CH 4) is: This is derived by following 5 general steps for writing Lewis dot structures. First, we need to... See full answer below.
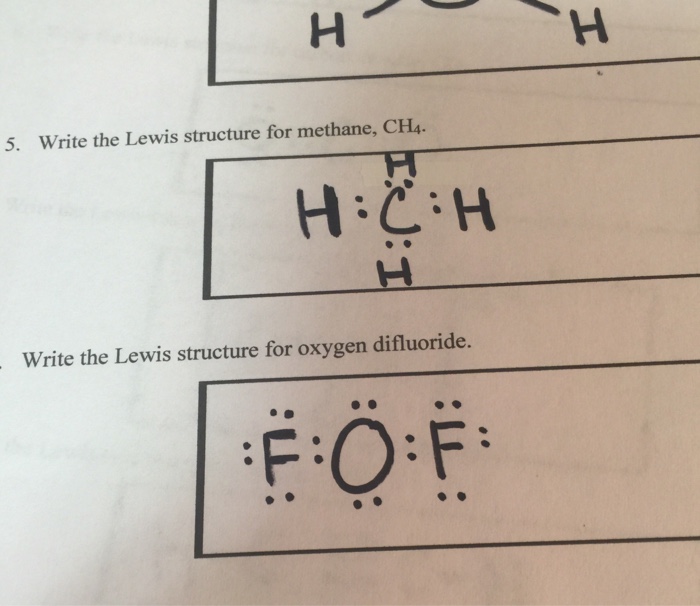
Lewis Structure of Methane (CH4)H|H--C--H|HorH..H : C : H..H. If the atom has a no charge (-/+ 0), the Lewis dot structure would be a capital "H" with one dot around it.
22 May 2019 — Rather, the structure simply reveals that the carbon atom has a complete octet of valence electrons in a methane molecule, that all bonds are ...
CH4 lewis's structure is very simple and easy to draw, it is made up of one carbon atom that takes the central position and four hydrogens that spread ...The formal charge of CH4: 0Total Valence electron for CH4: 8Molecular geometry/shape of CH4: TetrahedralBond angle: 109.5°
Answer: Well Carbon only has 4 valence electron, so it can bond at all four point. Hydrogen only has one valence electron and can only share one. Methane's (When it comes to hydrocarbons, "meth" stipulates one carbon, "ane" stipulates a single bond shared with hydrogens) molecular formula is CH4,...
Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) . Lewis dot dragram for methane: Methane, with molecular formula CH4, is shown. It is important to remember that Lewis valence dot diagrams are models that Methane is the main component of natural gas, and its chemical formula is CH4.










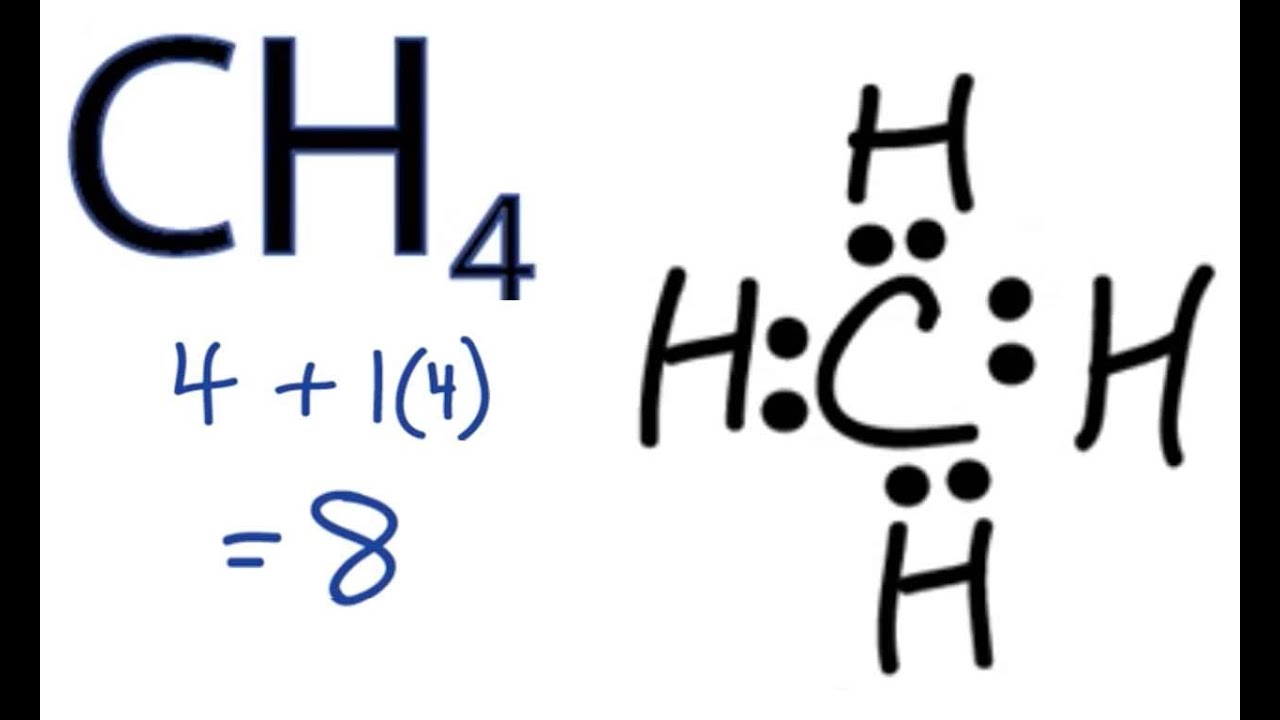

















0 Response to "44 lewis diagram for methane"
Post a Comment