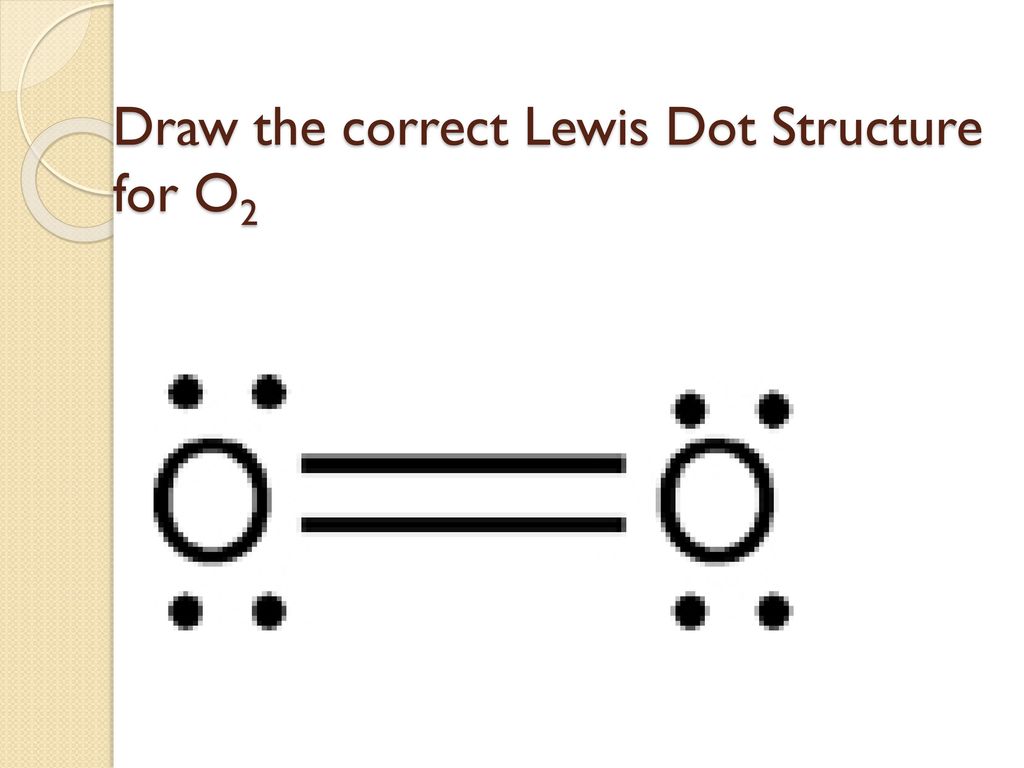
44 o2 lewis dot diagram
How to draw the Lewis Structure of Oxygen Gas - with explanation!Check me out: http://www.chemistnate.com
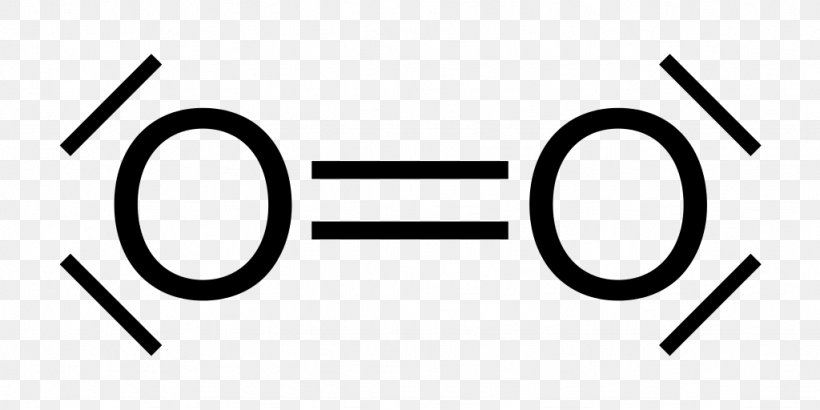
The diagram is drastically out of scale, as the relative size of the nucleus compared to the surrounding electrons is usually comparable to a pea in a stadium. O2 Properties The O 2 Lewis structure shows two oxygen atoms bonded in the same way to each other. It’s perfectly symmetric. Generally, small symmetric molecules are nonpolar.
A step-by-step explanation of how to draw the Lewis dot structure for O (Oxygen). I show you where Oxygen is on the periodic table and how to determine how ...

O2 lewis dot diagram
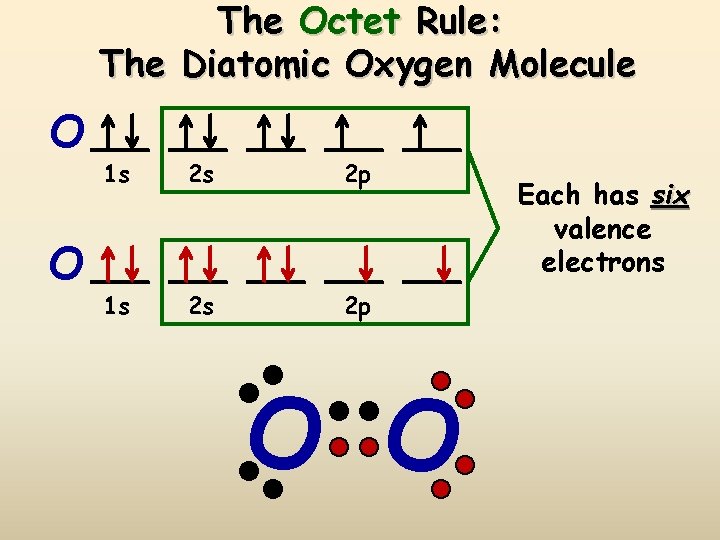
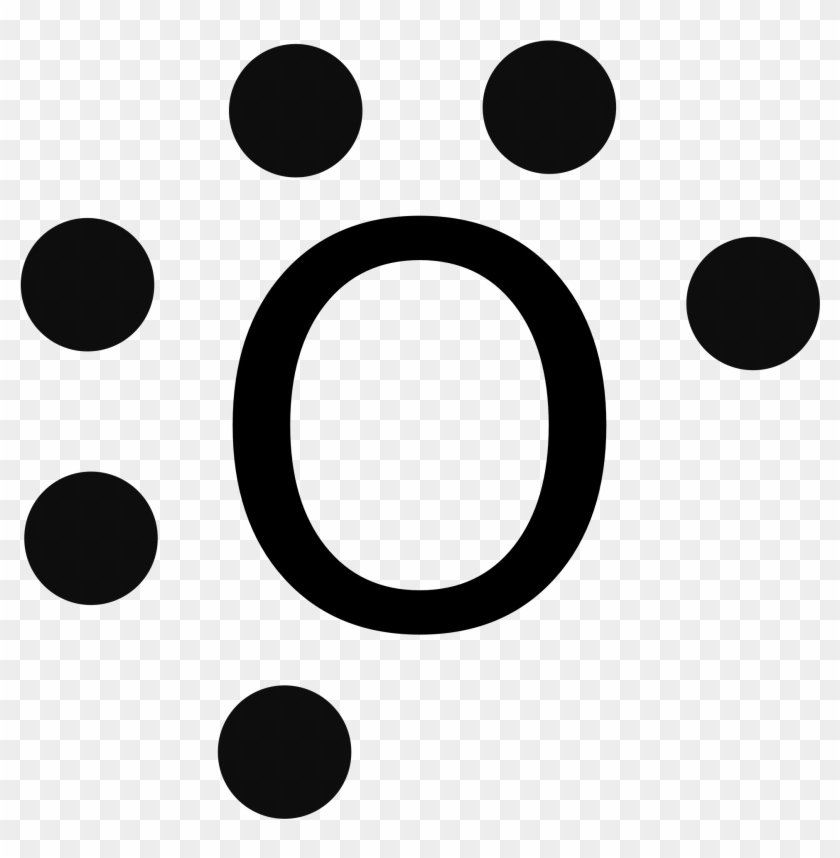
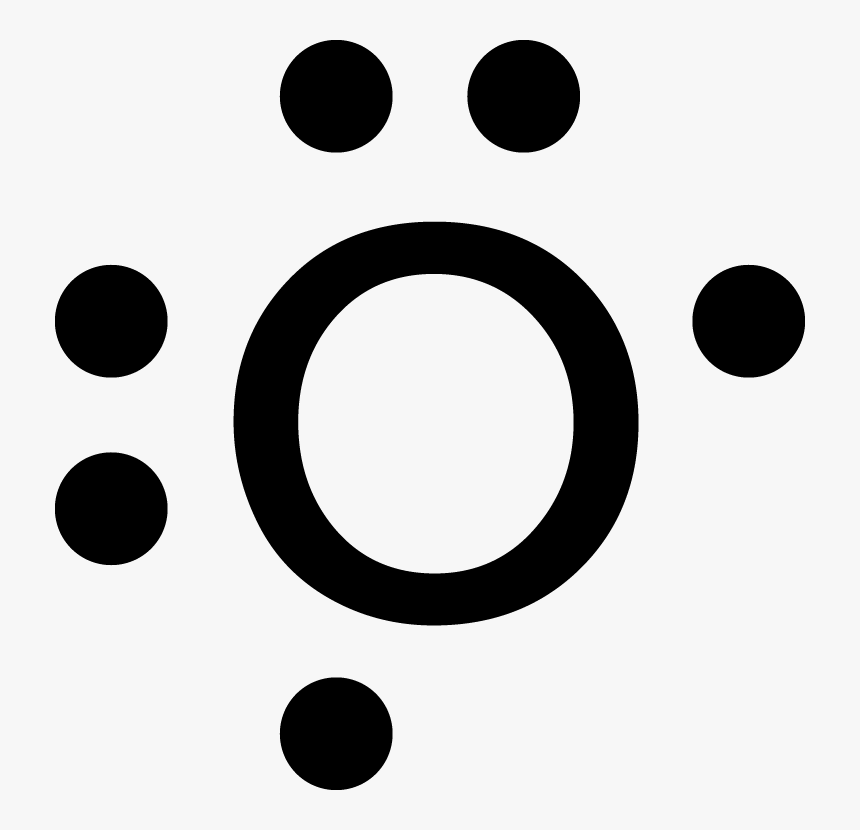
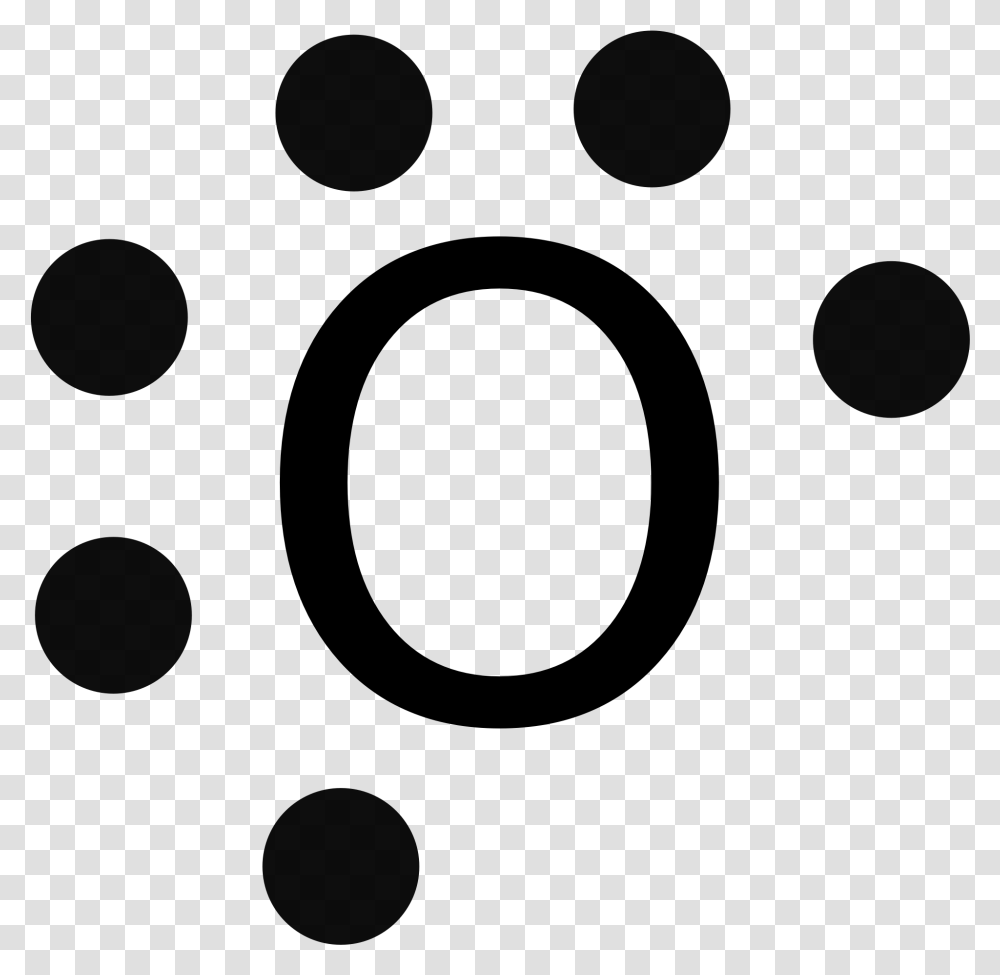
An element follows the octet rule to obtain a stable state. In case of oxygen atoms present in group 16 of the periodic table. It has six valence electrons with ...
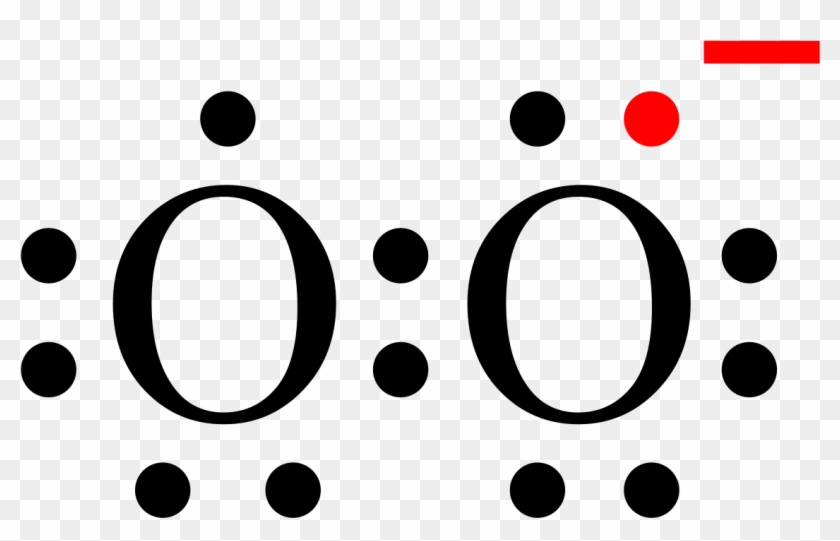
The Lewis diagram of O2 shows two oxygen atoms having twelve dots, of valence electrons. Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule.
A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Diatomic Oxygen).Note that Diatomic Oxygen is often called Molecular Oxygen or just Oxy...
O2 lewis dot diagram.
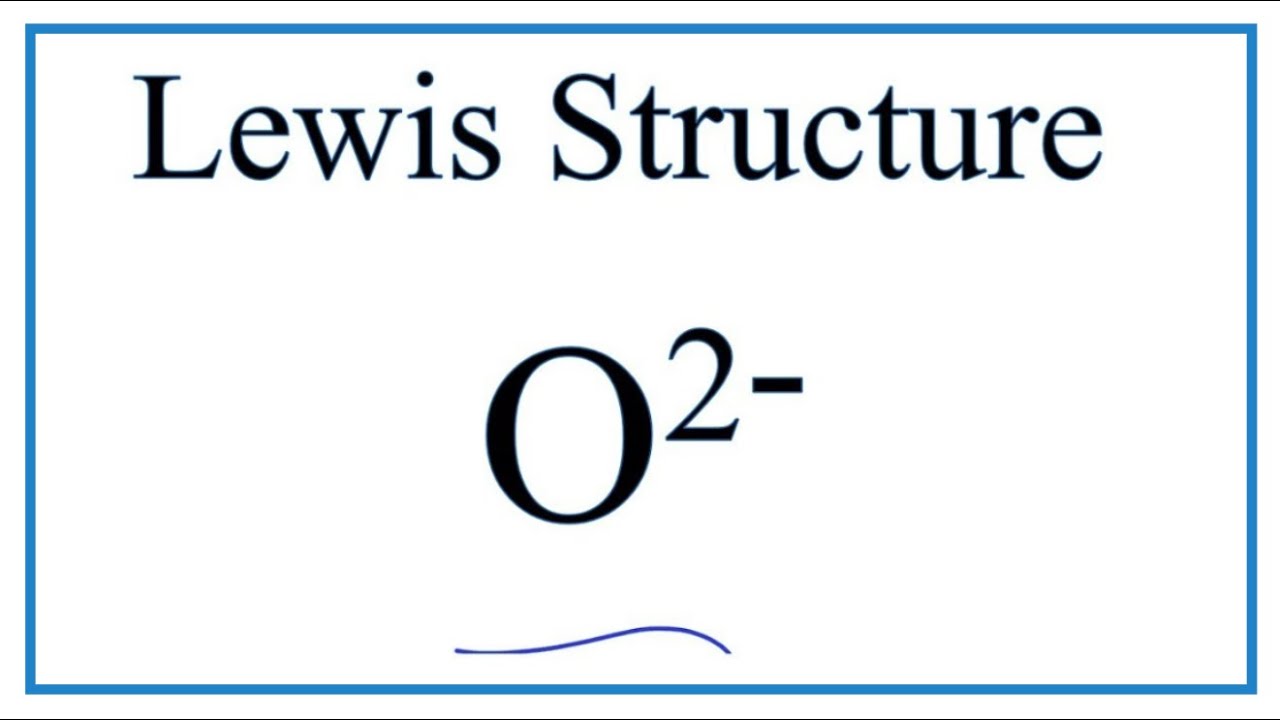
A step-by-step explanation of how to draw the O2- Lewis Dot Structure.For the O 2- structure use the periodic table to find the total number of valence elect...
Dioxygen (O2) is used in cellular respiration by many living organisms, used to create energy along with sugars. How To Interpret A Lewis Structure Lewis structures are diagrams that represent atoms and the bonds between them. The letters represent the atoms found within the molecule, with specific letters representing different elements.
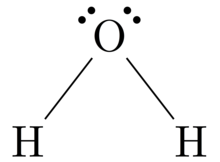
Lewis dot structures for the first few non-‐metals. The lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. Lewis dot diagrams use dots arranged around the atomic symbol to represent the electrons in the outermost energy level of an atom.
Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Transcript: This is the O2 2- Lewis structure.
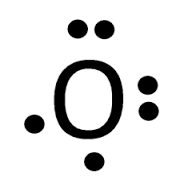
To write (any) Lewis structure, write up to 8 dots around the elements letter, going clockwise a quarter of a turn per dot. Oxygen is in the 6th column ( ...4 answers · 19 votes: Oxygen atoms have 6 valence electrons. They need a stable octet but forming one bond wouldn't ...
A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Oxygen Gas (Diatomic Oxygen)).For the O2 structure use the periodic table to find the t...



/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)












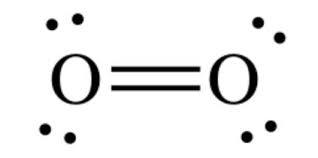








0 Response to "44 o2 lewis dot diagram"
Post a Comment