42 lewis dot diagram ch4
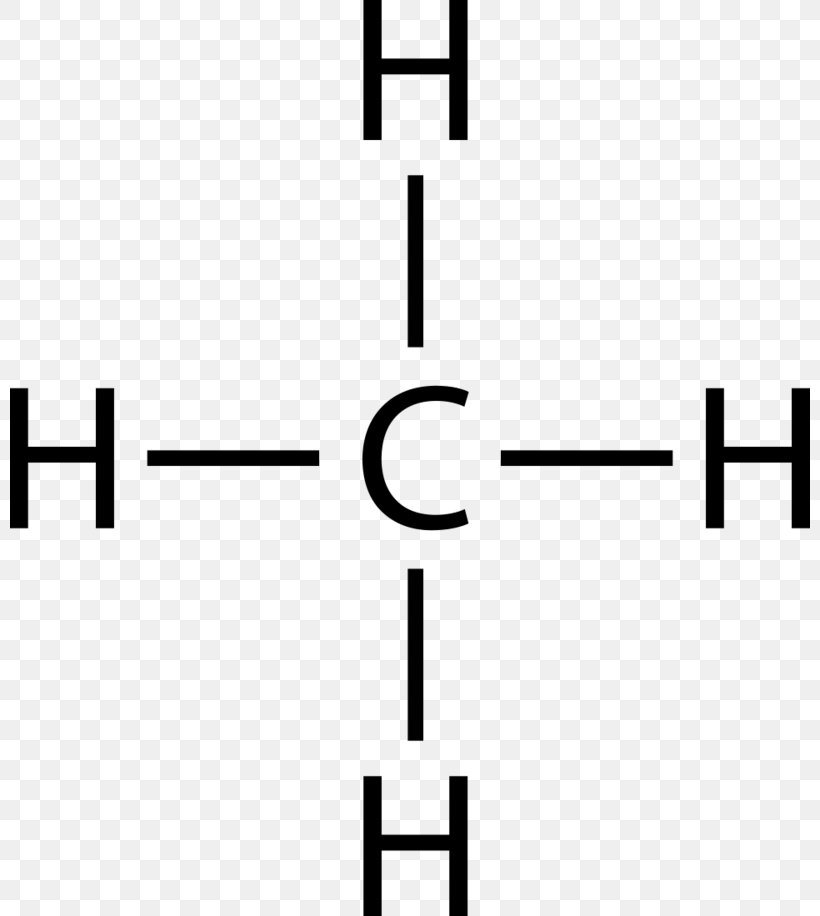
Lewis Structure of CH4. The lewis structure of carbon and hydrogen atom says- to form a single CH4 molecule, a total of eight valence electrons participate in the shared bonding to fulfill the need of eight more valence electrons. Here we will learn about how the lewis dot structure is drawn for CH4 molecule, step by step. CH4 Lewis Structure Lewis structure is the pictorial representation of the arrangement of valence shell electrons in the molecule, which helps us understand the atoms’ bond formations. The electrons that participate in bond formation are called the bonding pair of electrons, while those that don’t are known as nonbonding pairs of electrons.
Lewis structures, also known as Lewis dot diagrams, Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.The Lewis Dot Structure for CH4 - MakeTheBrainHappyLewis structure - Wikipedia

Lewis dot diagram ch4
Drawing the Lewis Structure for CH 4. For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single bonds.It's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. I am having trouble with a question about a lewis dot diagram. If phosphorus and bromine atoms formed a compound, what would the lewis dot diagram and chemical formula be? Would the chemical formula be: PBr? (I couldn't find the formula anywhere on the internet to confirm if the formula is simply PBr). If it is not simply PBr, why or what makes it something else? If it is just PBr, would the lewis diagram be a single bond? \-I think I did it correctly, just wanting to make sure. Answer: I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. I have drawn them above. The red one in the middle is the one Carbon, and the four yellow ones are Hydrogens. Now I have drawn the Valence Electrons. These are the blue dots next to the...
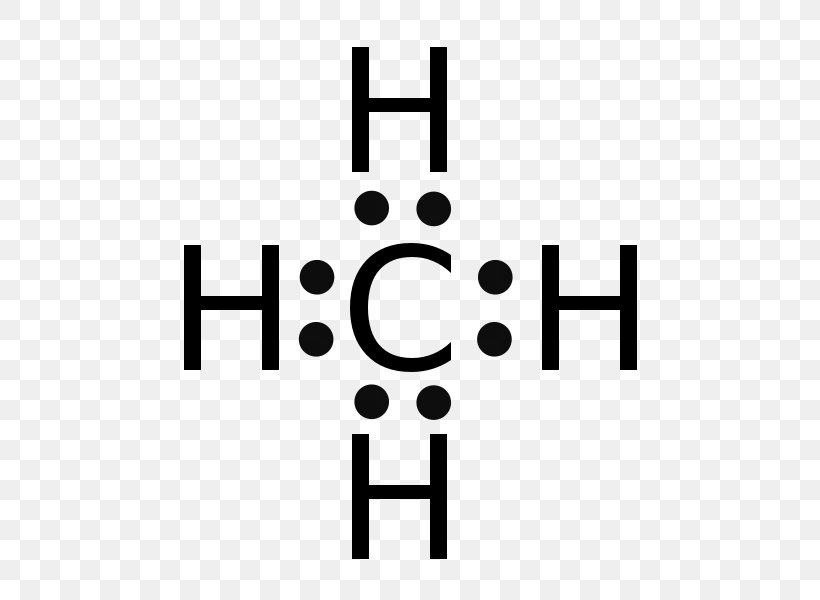
Lewis dot diagram ch4. If you have a link or image of one you can send to me that would be appreciated! How to draw lewis structure of CH4 (Methane)? CH4 lewis's structure is very simple and easy to draw, it is made up of one carbon atom that takes the central position and four hydrogens that spread around the central atom. There are no lone pair electrons present in the lewis dot structure of CH4. Shouldn't oxygen have two bonds? or does the negative outside the brackets signify that both oxygen and hydrogen gain that one electron? Am I thinking about this properly?! Methane lewis structure contains four C-H bonds. There are no lone pairs in the valence shells of carbon atom. Carbon atom is the center atom and it is very ...
How do I draw lewis dot diagrams? Do both elements need to have 8 electrons in the end? **This thread is no longer being updated, and has been replaced by:** # [Starship Development Thread #29](https://www.reddit.com/r/spacex/comments/rzi8hz/starship_development_thread_29/) [](/# Auto Sync Start) #### Quick Links[](https://www.flickr.com/photos/spacex/51369631902/) [^(NERDLE CAM)](https://www.youtube.com/watch?v=_HZCh2eGWEI) ^| [^(LAB CAM)](https://www.youtube.com/watch?v=HGb28t5TWtc&t=0s) ^| [^(SAPPHIRE CAM)](https://youtu.be/-MuTDO6ev9M) ^| [^(SENTINEL CAM)](https://... Dr. B. explains how to draw the Lewis dot structure for CH4(methane). The CH4Lewis Structure is one of the most frequently tested Lewis Structures. Note that hydrogen atoms always go on the outside of a Lewis dot structure. This is because they can share a maximum of two electrons. If anyone can give me an explanation or help me understand how it will look.
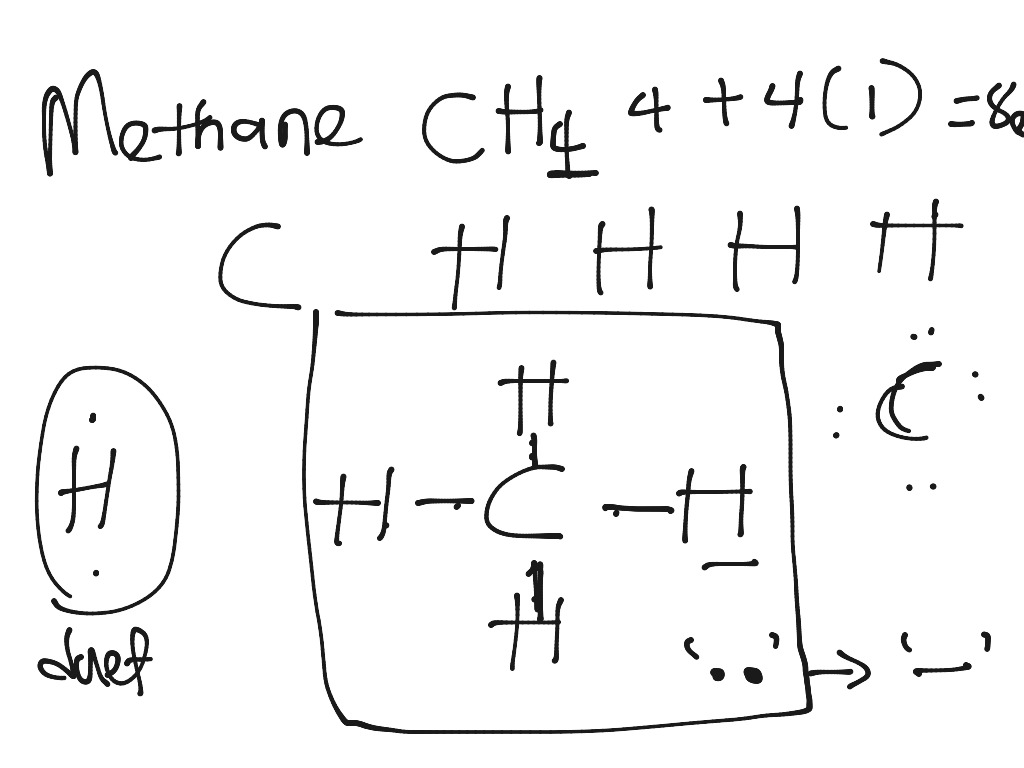
For methane CH4 Now the structure is complete: 20. 7. At the end, each atom should be surrounded by eight electrons (following the octet rule) Remember that H only needs 2 electrons 21. For methane CH4 • Carbon is surrounded by 8 electrons 22. For methane CH4 • Each hydrogen is surrounded by 2 electrons 23. Hello! Can someone please explain to me how to draw the Lewis diagrams for transition metals? I understand how to find the valence electrons based on the electron configuration. For elements 27+, they begin having more than 8 valence? Please help I am confused! (•‿•) I'll forever be in your debt 1 answerWhat is the correct Lewis dot diagram for CH4? · Solution · This is correct because it shows the each electron of C and hydrogen that are combining and shows ...
Draw Lewis dot diagram for the following. Methane (CH4) Maharashtra State Board HSC Science (General) 11th. Textbook Solutions 8028. Important Solutions 18. Question Bank Solutions 5552. Concept Notes ... Draw Lewis dot diagram for the following. Methane (CH 4) Advertisement Remove all ads.
Mar 10, 2018 · Lewis Dot Structure for CH4 #2 Find the number of “octet” electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 .May 03, · A step-by-step explanation of how to draw the Lewis Structure Oxygen Gas (Dioxygen). For the O2 Lewis structure, calculate the total number of valence electrons for the ...
The Lewis dot structure for CH 4 shows the number of valence electrons around each atom. Each dot represents a valence electron. The number of valence electrons . Write the symbol of the atom you are drawing the electron dot diagram for in the middle of your paper.
Lewis Dot Structure for CH4 (Methane) Properties of methane are described by Lewis Structure as cheaper natural gas than electricity. Methane, or CH4, is a natural gas that is relatively plentiful on earth, making it an environmentally effective source.
lewis dot diagram for sodium hypochlorite
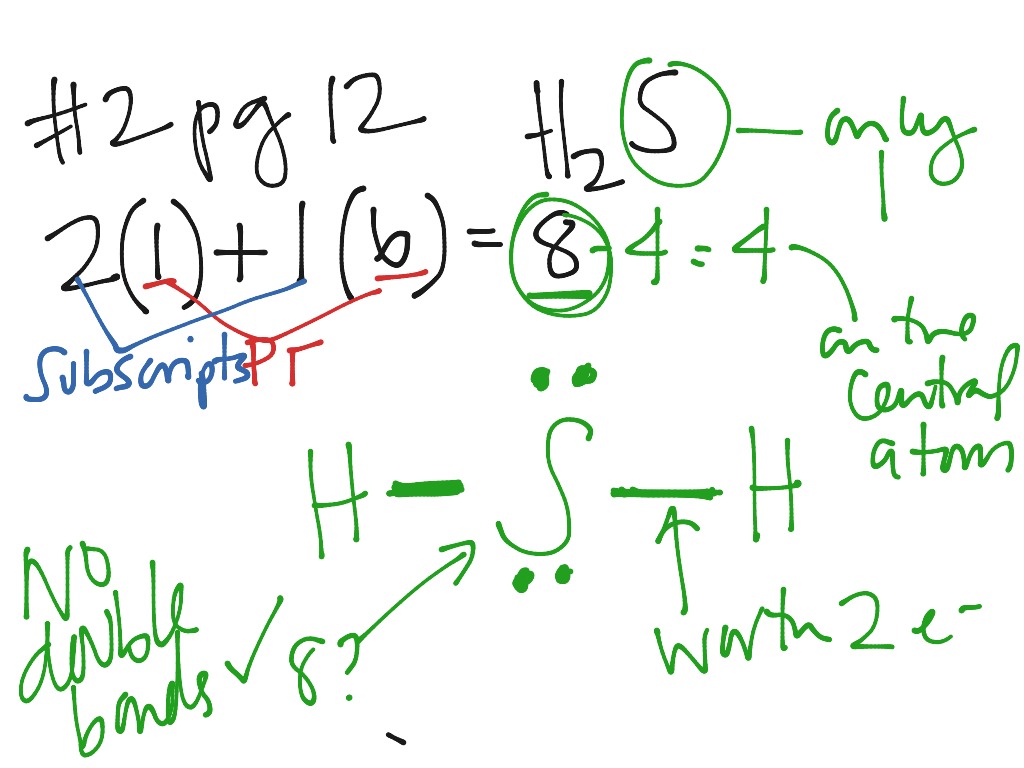
The dot structure of Na+1 is Na+1 . The dot structure of O-2 is O-2. Note that Na is in group 1 and should lose 1 electron while O is in group 6 and should gain 2 electrons. ionic compounds Make certain it's ionic: one atom must be from groups 1-3, the other from groups 4-7 (including H).
Jan 05, 2019 · Lewis dot structure of CH 4. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. C-4 H-1x4=4. Total=8. Put carbon in center and arrange hydrogen atoms on the diagramweb.nete electrons between carbon and hydrogen atoms. Dr. B. explains how to draw the Lewis dot structure for CH 4 (methane).
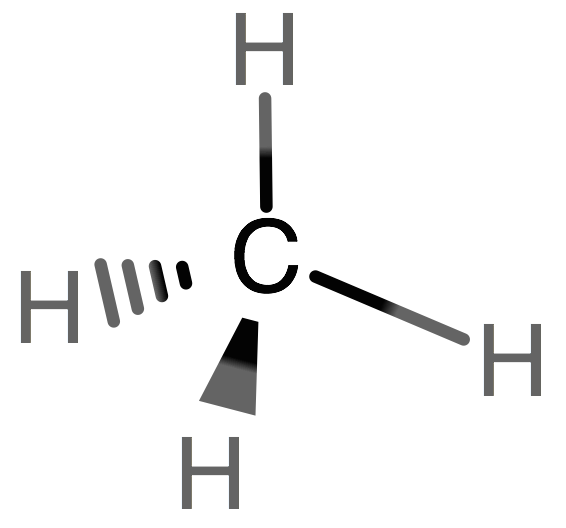
Methane, CH4, is a hydrocarbon. The structure is given below. The black sphere represents the carbon atom and the blue spheres represent the H atoms. If you make a pyramid using the H atoms, you will get a pyramid with 4 faces, all of whch are triangles. This is called a tetrahedral, and that is the formal name for the structure of methane.
Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) . Lewis dot dragram for methane: Methane, with molecular formula CH4, is shown. It is important to remember that Lewis valence dot diagrams are models that Methane is the main component of natural gas, and its chemical formula is CH4.
What is the correct Lewis dot diagram for CH4 ? 11th. Chemistry. Chemical Bonding and Molecular Structure. Basics of Chemical Bonding.
CH4 Lewis Structure & Molecular Geometry. Methane ( CH4) is a colourless, odourless, and highly combustible gas that is utilized to generate energy and heat houses all over the world. CH4 Lewis structure comprises two different atoms: Carbon and hydrogen. It is a nonpolar molecule with a bond angle of 109.5° degrees.
Recently we began a chapter in Chemistry where we had to find the Lewis Dot Structures of Molecules (I.e. using valence electrons, adding in bonds, lone pairs, etc.) and I simply cannot get it. The process and sequence to get to the finished product is confusing, which is unfamiliar to me, as I am someone who typically very easily grasps concepts. I would very much appreciate anyone who could shed some light on their ways of thinking, easier way to go about it, or other tips, as my teacher has e...
What is the Lewis dot structure of methane? The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3.
CH4 Lewis structure Methane electron dot structure is that type of diagram where we show the total 8 valence electrons of CH4 as dots or dots and dashes -In Lewis structureit is common that a bonding pair of two electrons can be shown by dash - or dots but a lone pair of two electrons is shown by dots. A Estructura electrónica de Lewis.
The quiz will present you with different compounds and then ask you to identify the correct Lewis dot diagram. It will also ask you to identify the various conventions related to Lewis dot ...
The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3.
A step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use the periodic table to find the total number of vale...
Can someone please help me figure out how to draw the Lewis dot diagram for a Na- anion? Thank you.
Molecular Orbital diagram of CH4 The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with four 1s atomic orbitals of the hydrogen.
Lewis Dot Structures can be produced by following a sequence of steps. Let's produce a Lewis Dot Structure for: NH 4 + (the ammonium ion). Step 1: Count valence electrons: N = 5 4 x H = 4 x 1 = 4 "+" = -1 Total = 5+4-1= 8 electrons = 4 bonds and lone pairs. Step 2:!Arrange the atoms (identify a central atom, if possible).
A single Lewis structure can be used to represent the bonding in CH4.There are 2 equivalent Lewis structures for nitryl chloride where the double bond to oxygen can be placed on either of the ...
Lewis Structures for CH4. Step-by-step tutorial for drawing the Lewis Structure for CH4. The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the . I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane.
Silicon tetrachloride (SiCl4) lewis dot structure, molecular geometry, polar or non-polar, hybridization Home > Chemistry Article > SiCl4 lewis structure and its molecular geometry Silicon tetrachloride is an inorganic compound that appears as a colorless liquid with a pungent odor having the chemical formula SiCl4.
A Lewis dot structure is a drawing of a molecule. The drawing only "works" f0r stable molecules that actually exist. So it's a nice tool to explore how atoms bond into more complex substances. A Lewis dot structure is also called a Lewis structure, a Lewis dot diagram, an electron dot structure, or a dot diagram.
[](/# Auto Sync Start) #### Quick Links[](https://www.flickr.com/photos/spacex/51369631902/) [^(NERDLE CAM)](https://www.youtube.com/watch?v=_HZCh2eGWEI) ^| [^(LAB CAM)](https://www.youtube.com/watch?v=HGb28t5TWtc&t=0s) ^| [^(SAPPHIRE CAM)](https://youtu.be/-MuTDO6ev9M) ^| [^(SENTINEL CAM)](https://youtu.be/zPkIZYw5O98) ^| [^(ROVER CAM)](https://youtu.be/5HpgJJ1FwTc) ^| [^(PLEX CAM)](https://youtu.be/H_Y__hUYrQ4) ^| [^(NSF STARBASE)](https://youtu.be/mhJRzQsLZGg) ^| [^(MORE LINKS)](/r/sp...
The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the bond has a full valence, with carbon having access to eight electrons and each hydrogen having access to two ( this is why hydrogen only needs two).
How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use...
CH4 has a tetrahedral shape. Carbon is the central atom, and there are 4 Hydrogen atoms surrounding it. There are 4 covalent bonds surrounding it. These bonds ...2 answers · 1 vote: I would be lazy and look it up on the internet. But seriously, you have an electron pair ...
Answer: I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. I have drawn them above. The red one in the middle is the one Carbon, and the four yellow ones are Hydrogens. Now I have drawn the Valence Electrons. These are the blue dots next to the...
I am having trouble with a question about a lewis dot diagram. If phosphorus and bromine atoms formed a compound, what would the lewis dot diagram and chemical formula be? Would the chemical formula be: PBr? (I couldn't find the formula anywhere on the internet to confirm if the formula is simply PBr). If it is not simply PBr, why or what makes it something else? If it is just PBr, would the lewis diagram be a single bond? \-I think I did it correctly, just wanting to make sure.
Drawing the Lewis Structure for CH 4. For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single bonds.It's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.

























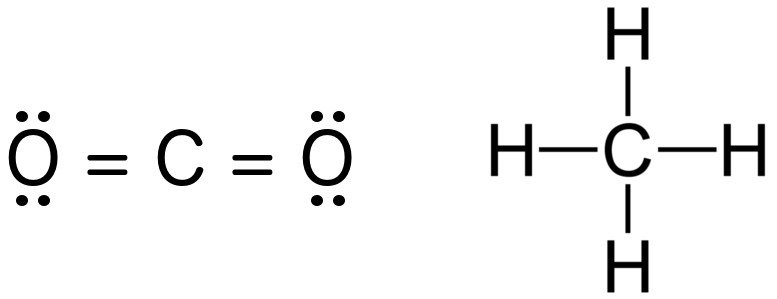
0 Response to "42 lewis dot diagram ch4"
Post a Comment