42 lithium molecular orbital diagram
Answer: The manifold I remember for n=2 diatomics is single-single-double-single-double-single. Repeat that over and over again. It's the sequence of the energy levels in the molecular orbital scheme for n-2 diatomics. after that, just count the number of electrons and put them into the energy le... The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 2.7.8). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons.
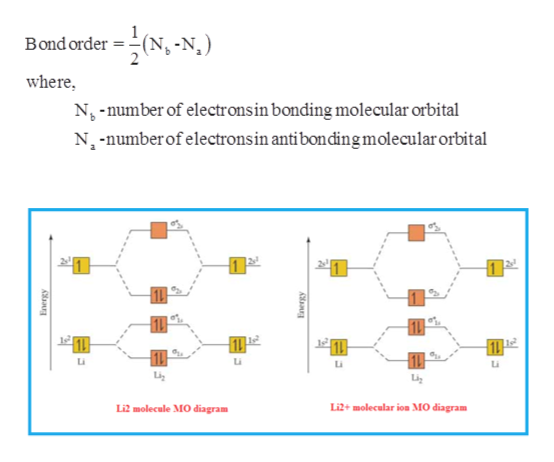
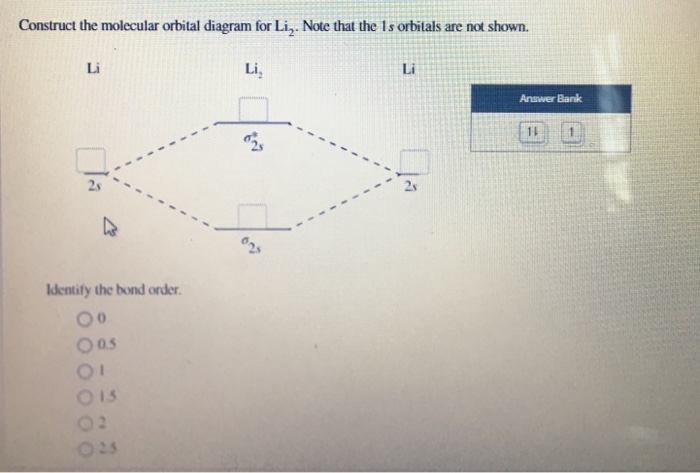
You can use the following formula for the bond order. B O = N b − N a 2. Here, B O, N b. and. N a. represents the bond order, the number of electrons in bonding molecular orbitals and the number of electrons in antibonding molecular orbitals respectively. Complete step by step answer: The atomic number of lithium is 3.

Lithium molecular orbital diagram
Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ... Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ... Molecules Lithium molecule (Li2): The formation of Lithium (Li) molecule is made possible by the overlapping of two lithium atoms and each consisting of electronic configuration 1s2 2s1. So, we are having a total of 6 electrons which needs to be accommodated in four molecular orbitals, namely, σ1s, σ*1s, σ2s, and σ*2s.
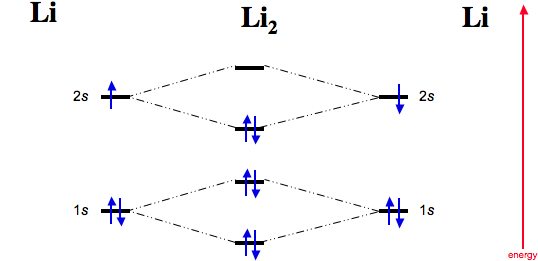
Lithium molecular orbital diagram. 22 Dec 2020 · 1 answer1. Electronic configuration of Li atom – 1s · 2. Electronic configuration of Li2 molecule is σ1s2 σ*1s2 σ2s · 3. Bond order = Nb−Na2 N b − N a 2 ... The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. The molecule . Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = (). B.O = 1. A molecular orbital diagram, Beryllium has an electron configuration 1s 2 2s 2, so there are again two electrons in the valence level. However, the 2s can mix with the 2p orbitals in diberyllium, whereas there are no p orbitals in the valence level of hydrogen or helium. The orbital filling diagram of boron. I skipped past beryllium because I ... Lithium hydride — Of the four electrons in lithium and hydrogen, two are retained in the lithium 1s orbital, and the two remaining ones reside in the σ ...Molecular Orbital Diagrams · Dihydrogen · Dihelium
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Orbital diagram for lithium. the atomic formula of lithium is 3 . so the electronic concentration will be 2,1 . this means that there will be 2 electrons in first shell n 1 electron in second shell. How to draw Molecular Orbital Diagram of Lithium molecule (Li 2) ? According to the molecular orbital theory ,the molecular orbital diagram of lithium molecule is shown below: The 2 electrons are present in σ 2s orbitals . The anti bonding molecular orbital σ*2 s is empty . Thus Number of electrons in BMO – Number of electrons in ABMO 2- The combination of two lithium atoms to form a lithium molecule, Li 2, is analogous to the formation of H 2, but the atomic orbitals involved are the valence 2s orbitals. Each of the two lithium atoms has one valence electron. ... molecular orbital diagram: visual representation of the relative energy levels of molecular orbitals.
We are being asked to determine the molecular-orbital diagram for a chain containing six lithium atoms.. Recall: In the molecular orbital theory, electrons are seen as being delocalized or spread out over a molecule instead of concentrated in a covalent bond.. A bonding orbital is a region of high electron density between nuclei where a bond forms; An anti-bonding orbital is a region that has ... The diagram shows how the molecular orbitals in lithium hydride can be related to the atomic orbitals of the parent atoms. One thing that makes this diagram look different from the ones we have seen previously is that the parent atomic orbitals have widely differing energies; the greater nuclear charge of lithium reduces the energy of its 1 s ... 8 - Drawing Molecular Orbital Diagrams. Abstract (TL;DR) Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds. Depending on if it is a homonuclear case, where the bonding atoms are the same, or a heteronuclear case, where the bonding atoms are ... Figure 5.34 This is the molecular orbital diagram for the homonuclear diatomic Be 2 +, Be 2 +, showing the molecular orbitals of the valence shell only. The molecular orbitals are filled in the same manner as atomic orbitals, using the Aufbau principle and Hund's rule.
Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds.
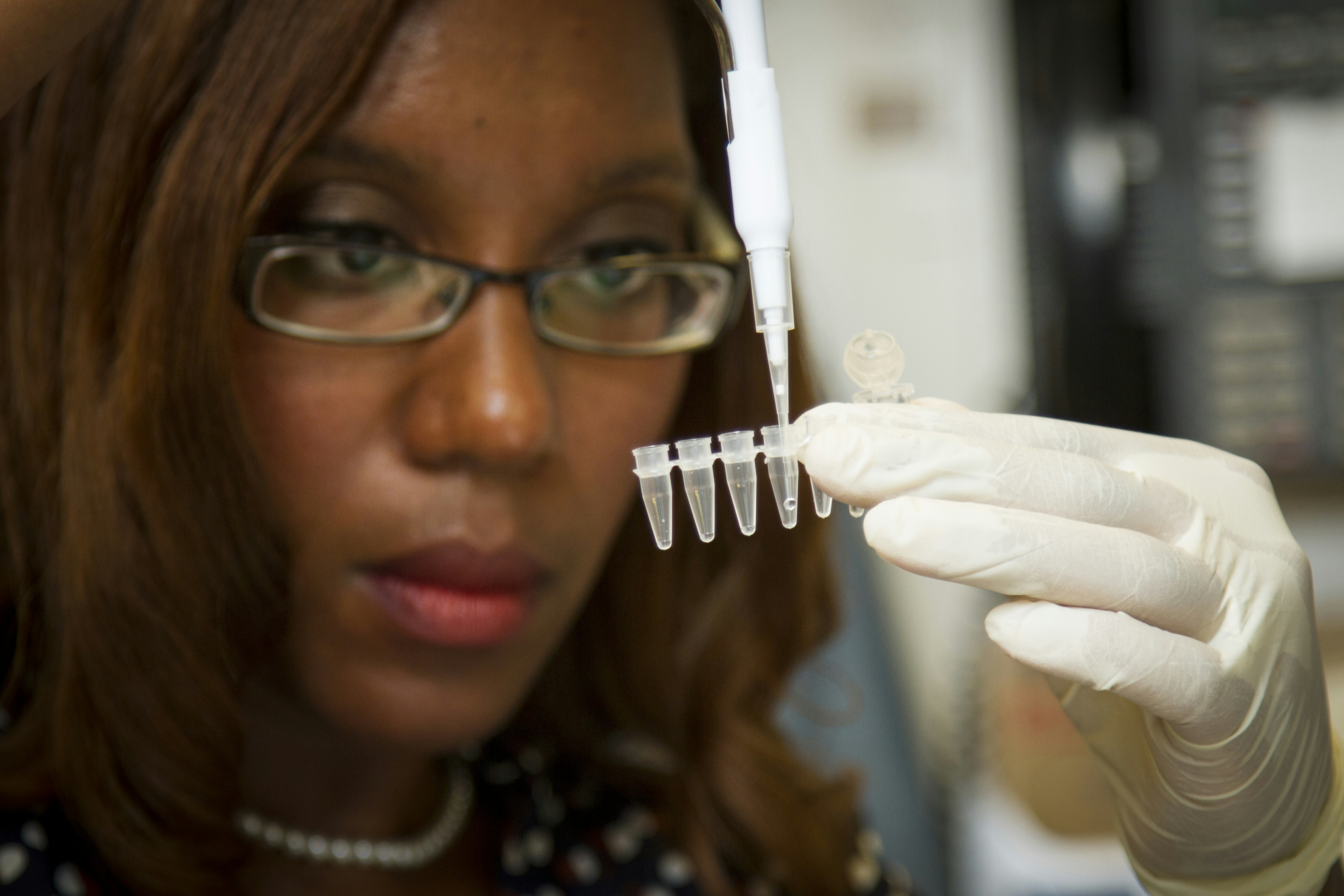
Chanelle Case Borden, Ph.D., a postdoctoral fellow in the National Cancer Institute's Experimental Immunology Branch, pipetting DNA samples into a tube for polymerase chain reaction, or PCR, a laboratory technique used to make multiple copies of a segment of DNA.
Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
Orbital diagram for lithium? the atomic formula of lithium is 3 . so the electronic concentration will be 2,1 . this means that there will be 2 electrons in first shell n 1 electron in second ...
Molecular Orbital Diagram of Lithium Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET....
Formation of the molecular orbitals also changes the energy level of the core orbitals even though these do not participate appreciably in the bonding. This is because what I call "reverse shielding" occurs. In a lithium atom in the ground state, there are of course two 1s electrons and one 2s electron.
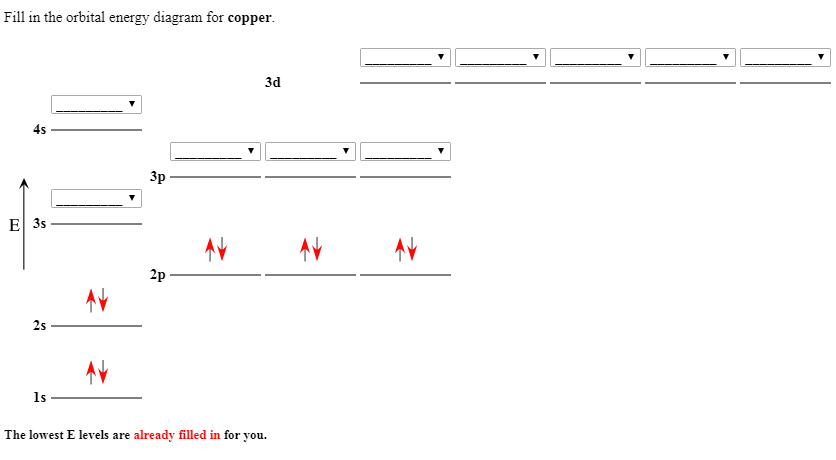
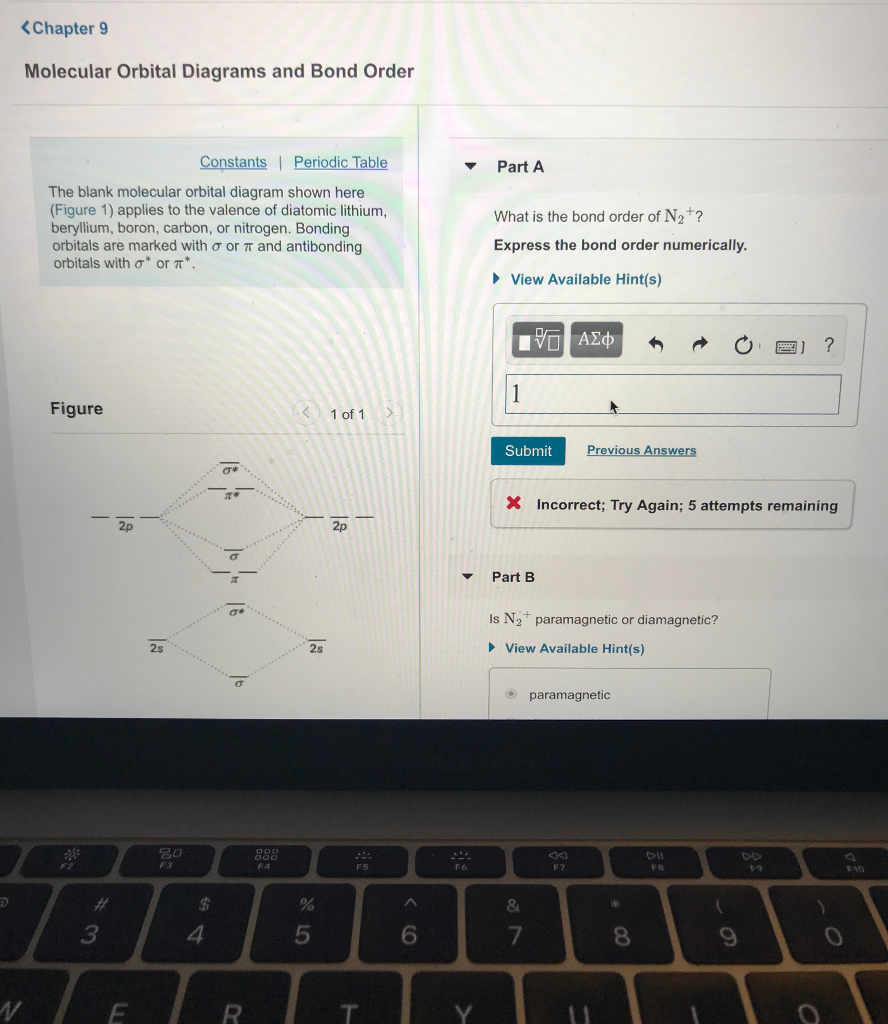
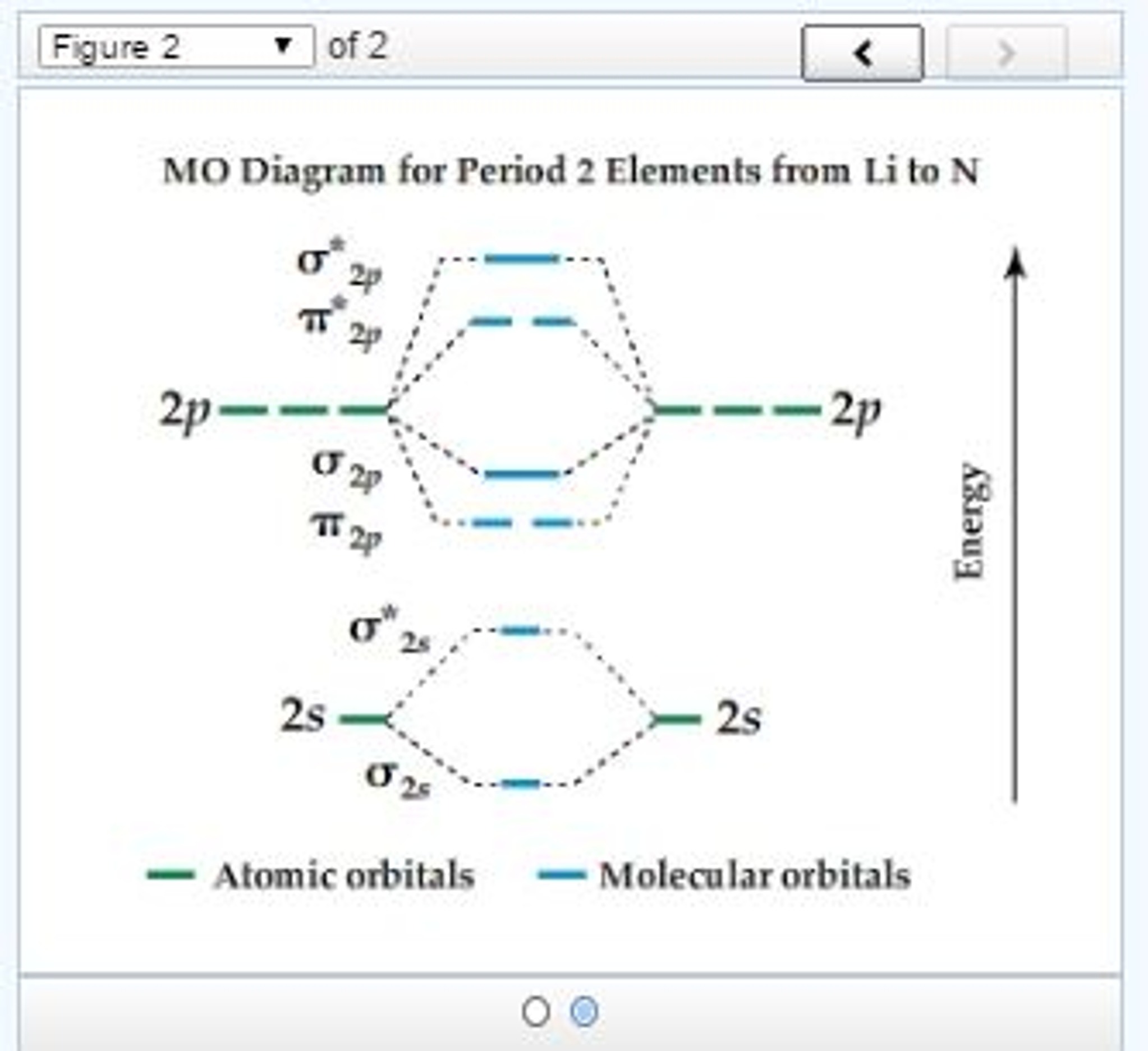
Molecular Orbital Diagrams and Bond Order Constants Periodic Table Part A The blank molecular orbital diagram shown here (Figure 1) applies to the valence of diatomic lithium, beryllium, boron carbon, or nitrogen. Bonding orbitals are marked with σ or π and antibonding orbitals with σ* or π*.

The National Cancer Institute's Natural Products Branch at the Frederick National Laboratory for Cancer Research is the largest program to collect materials worldwide from marine, plant, and microbial sources so they may be studied for possible medical uses. The fermentation lab grows fungal and bacterial cultures in liquid media. These materials are extracted using organic solvents, and eventually tested in the NCI cancer screens.
The lithium 1sorbital is the lowest-energy orbital on the diagram. Because this orbital is so small and retains its electrons so tightly, it does not contribute to bonding; we need consider only the 2sorbital of lithium which combines with the 1sorbital of hydrogen to form the usual pair of sigma bonding and antibonding orbitals.
Molecular orbitals: Orbitals that span two or more atoms. These are constructed by overlapping atomic orbitals (AOs) which match in symmetry and size. In principle, To construct MO diagram of a any Molecule, first, set up ... exists in gas phase over metallic lithium.
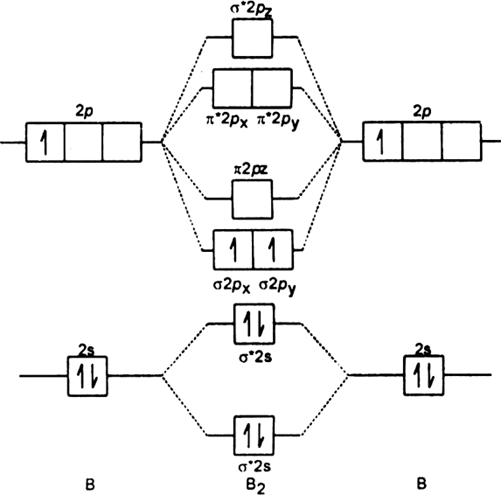
This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation.
Lithium belongs to the group 1 ( alkali metals) , period 2 s-block element of the periodic table . Lithium is represented by the symbol "Li". The atomic number of Lithium (Li ) is 3 and the mass number is 6.941. ... How to draw Molecular Orbital Diagram of Li2 ,Li 2+ , Li2 – | Simplest Trick – Chemistry.
Transcribed image text: Chapter9 Molecular Orbital Diagrams and Bond Order Periodic Table Part A The blank molecular orbital diagram shown here (Figure 1) applies to the valence of diatomic lithium, beryllium, boron, carbon, or nitrogen. Bonding orbitals are marked with ơ or and antibonding orbitals with σ* or π* What is the bond order of N2+? Express the bond order numeric
Molecular orbital energy level of Li2. diagram; the MOs go between them. Consider the MO diagram for Li2. This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital.
Molecular Orbital energy level Diagrams of Li2 according to the molecular orbital theory explains the bond order and magnetic properties of lithium molecule....
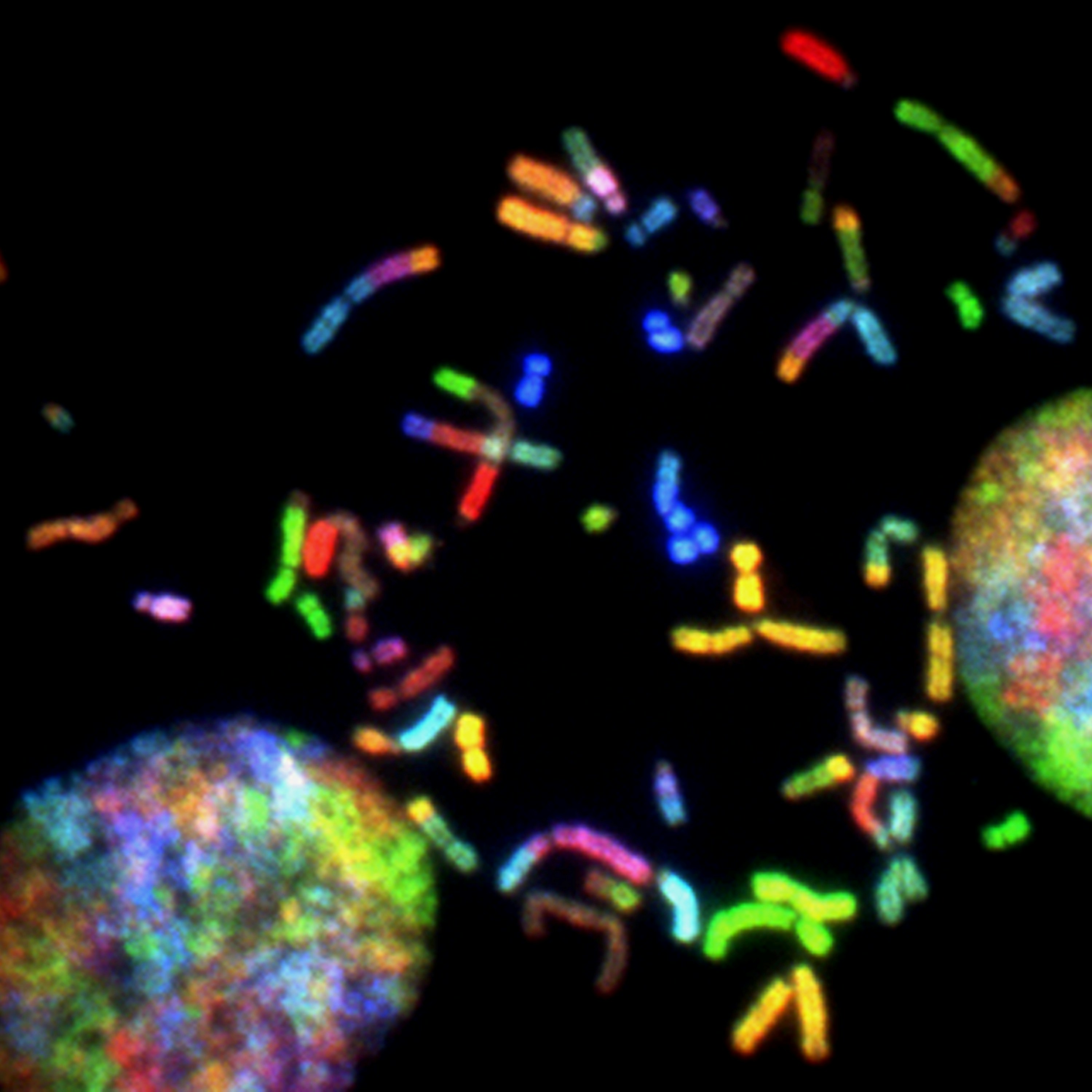
Brain Cancer Chromosomes. Chromosomes prepared from a malignant glioblastoma visualized by spectral karyotyping (SKY) reveal an enormous degree of chromosomal instability -- a hallmark of cancer. Created by Thomas Ried, 2014
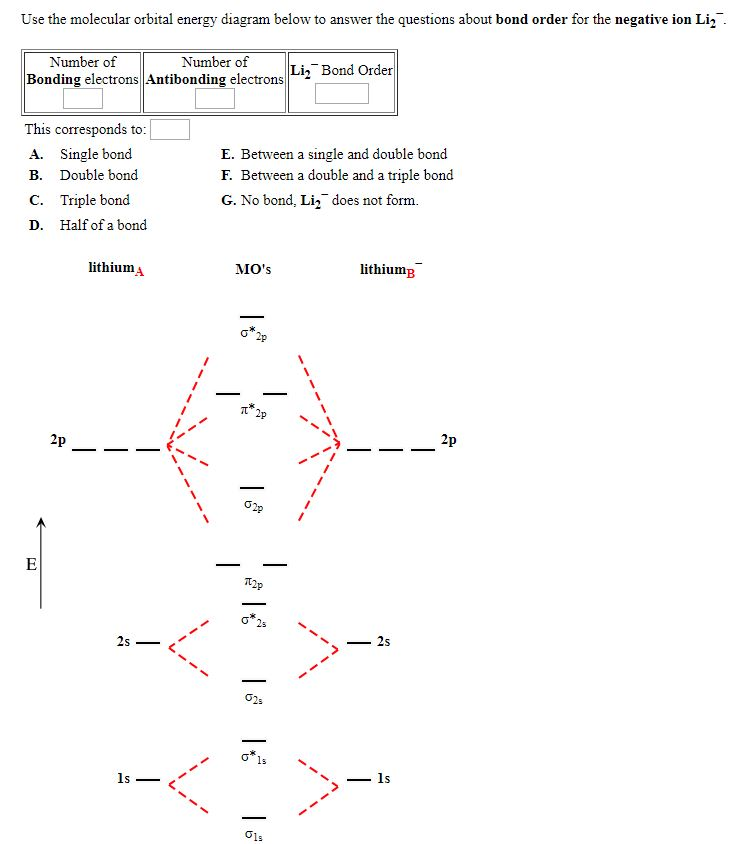
Category: science chemistry. 4.9/5 (1,280 Views . 33 Votes) A molecular structure that results in unpaired electrons in degenerate MOs, like O2, is called paramagnetic. Li2 has all of its electrons neatly paired up in the orbitals. Li2 is diamagnetic. Find out all about it here.
Molecules Lithium molecule (Li2): The formation of Lithium (Li) molecule is made possible by the overlapping of two lithium atoms and each consisting of electronic configuration 1s2 2s1. So, we are having a total of 6 electrons which needs to be accommodated in four molecular orbitals, namely, σ1s, σ*1s, σ2s, and σ*2s.
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ...

Chanelle Case Borden, Ph.D., a postdoctoral fellow in the National Cancer Institute's Experimental Immunology Branch, pipetting DNA samples into a tube for polymerase chain reaction, or PCR, a laboratory technique used to make multiple copies of a segment of DNA.
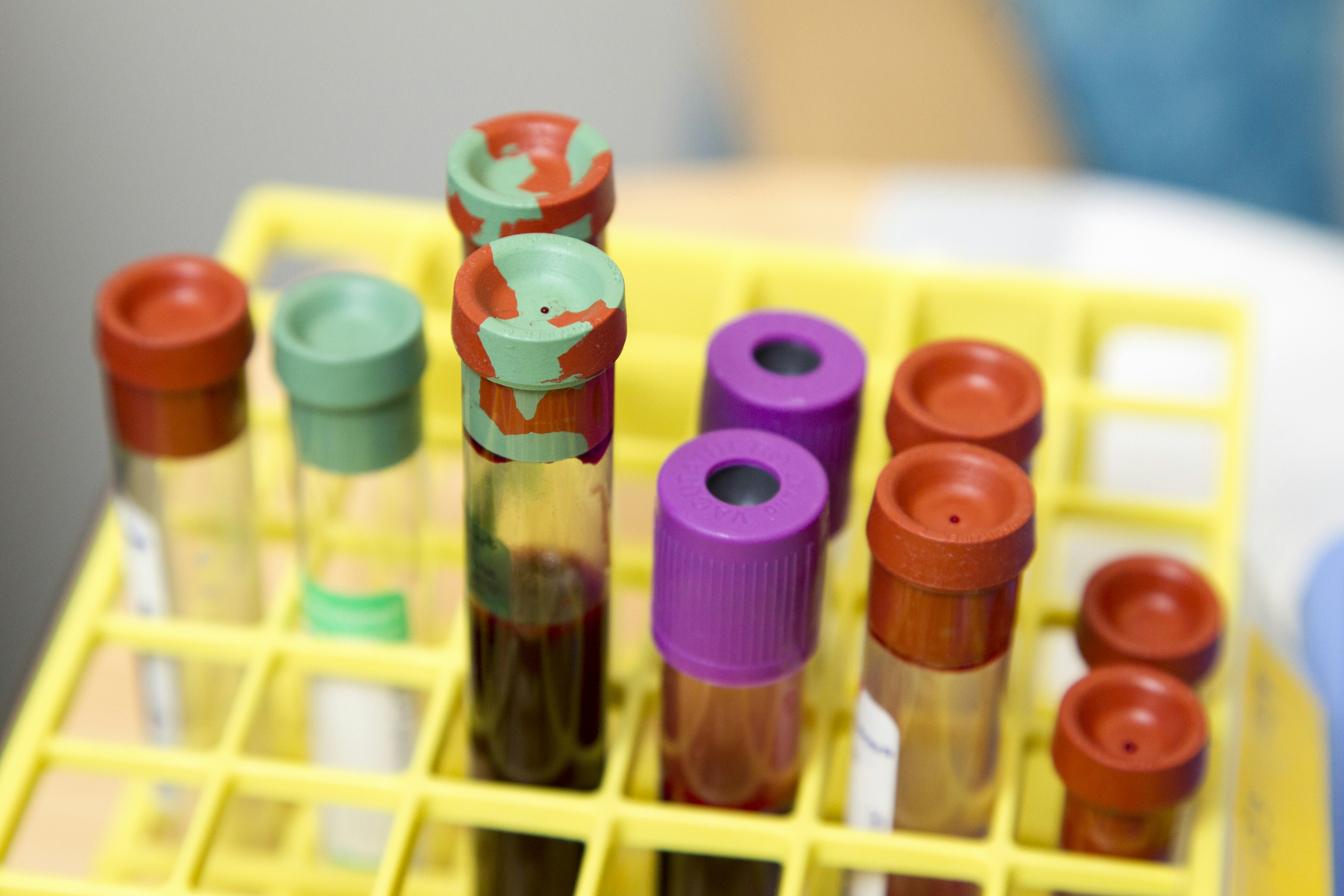
Vials of Blood. Vials of blood taken in the course of patient care at the National Institutes of Health Clinical Center in Bethesda, Maryland. Test tubes. Blood test. Creator: Daniel Sone

DNA Genotyping and Sequencing. A technician reviews data from high-throughput DNA genotyping and sequencing at the Cancer Genomics Research Laboratory, part of the National Cancer Institute's Division of Cancer Epidemiology and Genetics (DCEG).


















0 Response to "42 lithium molecular orbital diagram"
Post a Comment