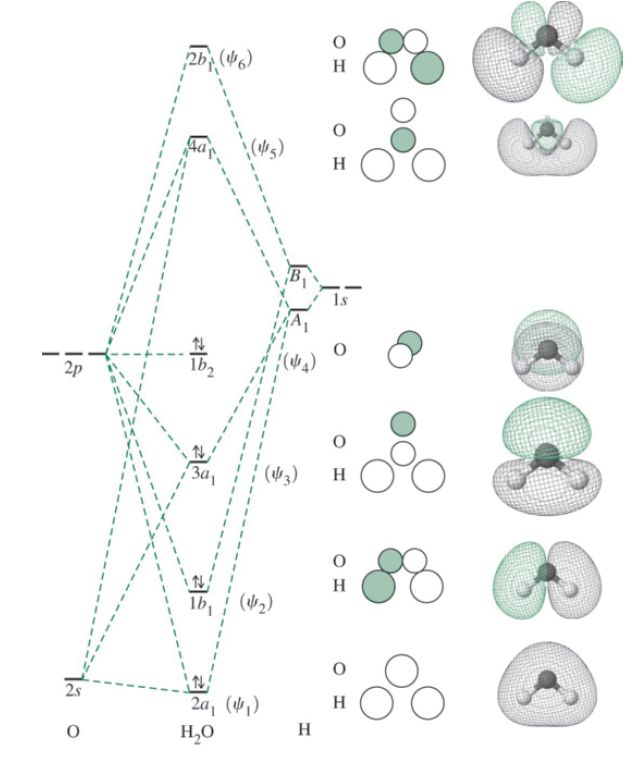
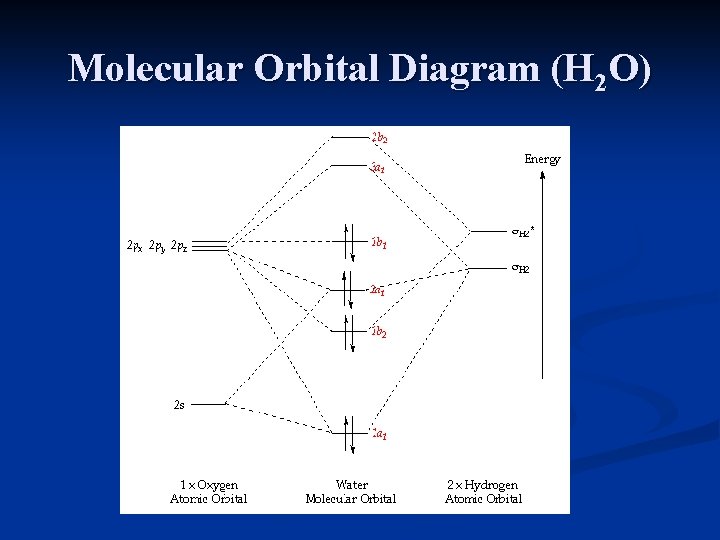
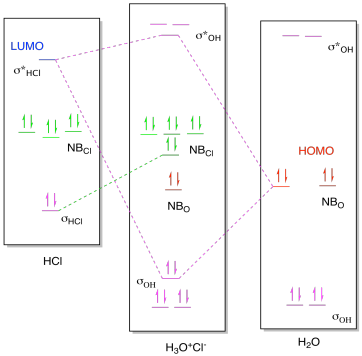
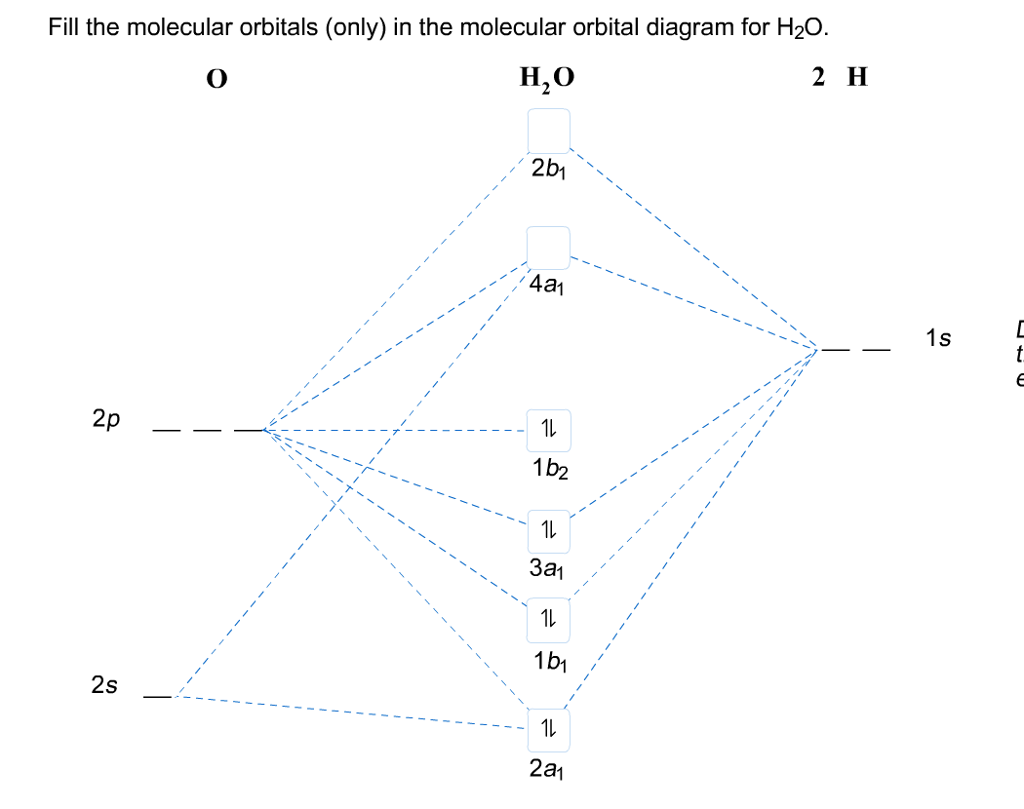
43 h20 molecular orbital diagram
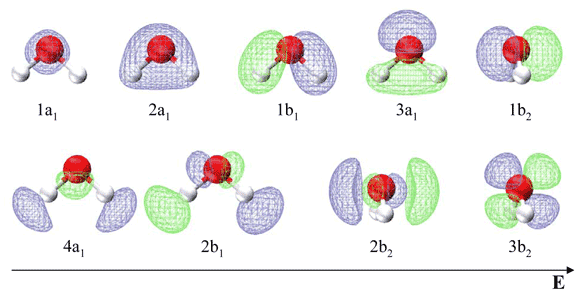
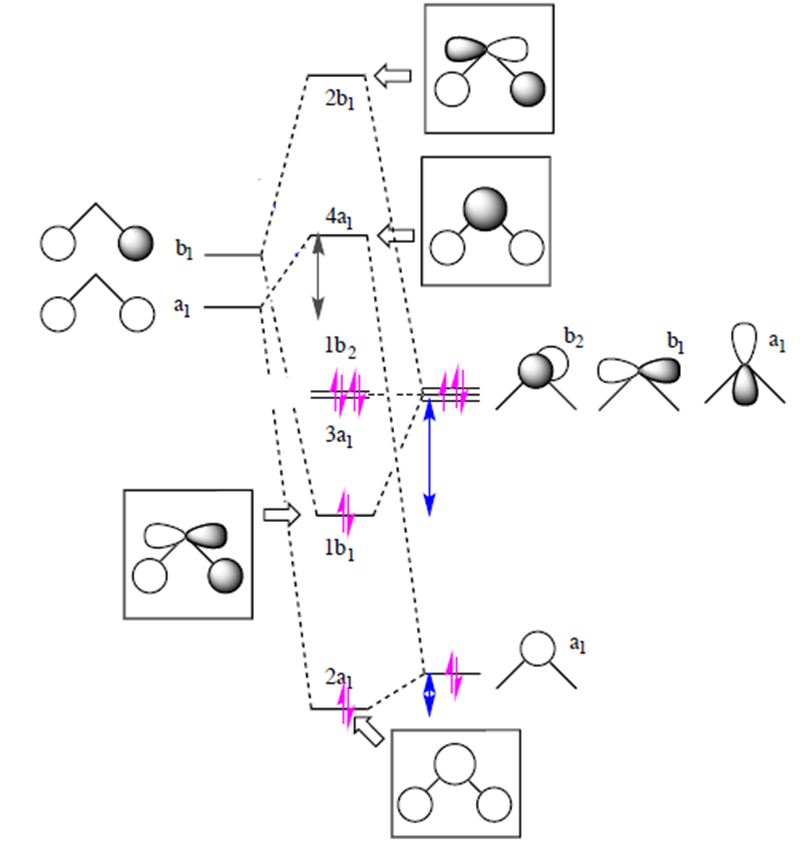
Description. H2O-MO-Diagram.svg. English: MO diagram of water. Vectorized, simplified and corrected from File:Diagramme AH2.png. Quantitative calculations show bonding character in both the 3a 1 and 2a 1 levels. Date. 21 May 2015. Source. Own work. Molecular Orbitals for Water (H 2 O) The five occupied and the lowest three unoccupied molecular orbitals of the isolated molecule (1a 1) 2(2a 1) 2(1b 2) 2(3a 1) 2(1b 1) 2 were calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set (experimental data is given in [1289]). They are set out with the lowest
An advanced molecular orbital diagram of H2O (water) for the inorganic or physical chemistry student.

H20 molecular orbital diagram
equivalent bond orbitals and sets of lone-pair orbitals. In H20, for example, there are two such sets, each with two members. In general there is an element of arbi-trariness in the definition of these sets, owing to the possibility of forming them from a linear combination of molecular orbitals of the same symmetry. Suppose, for About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... 6.2: Molecular Orbital Theory for Larger (Polyatomic) Molecules. We can extend the method we used for diatomic molecules to draw the molecular orbitals of more complicated, polyatomic molecules (molecules with more than two atoms). To combine several different atoms in a molecular orbital diagram, we will group orbitals from different atoms ...
H20 molecular orbital diagram. H20 Molecular Orbital Diagram. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals. General procedure for simple molecules that contain a central atom: build group orbitals using the outer atoms, then interact the group orbitals. Our goal is to apply the principles of quantum mechanics and electronic structure theory to address problems in physical, organic, inorganic, and biological chemistry. High performance computers are used to solve the complex equations describing the system of interest, yielding predictions of structures, bonding, energetics, reactivity, and other physical properties Orbitals of same symmetry and similar energy levels can then be mixed to form a new set of molecular orbitals with bonding, nonbonding, and antibonding characteristics. In the simple MO diagram of H 2 O , the 2s orbital of oxygen is mixed with the premixed hydrogen orbitals, forming a new bonding (2a1) and antibonding orbital (4a1). Consider the molecular orbital diagram for H20 and respond to the following questions: (a) (1) What is the total number of the formally bonding and antibonding orbitals in H2O? Answer: (b) (1) List all oxygen orbitals involved in bonding in H20. Answer: (c) (2) Name the oxygen orbitals that would not have been involved in bonding, if the ...
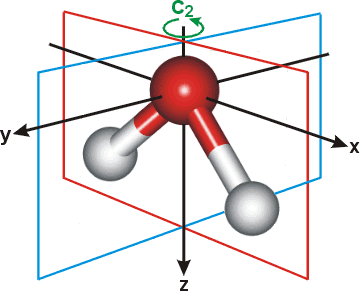
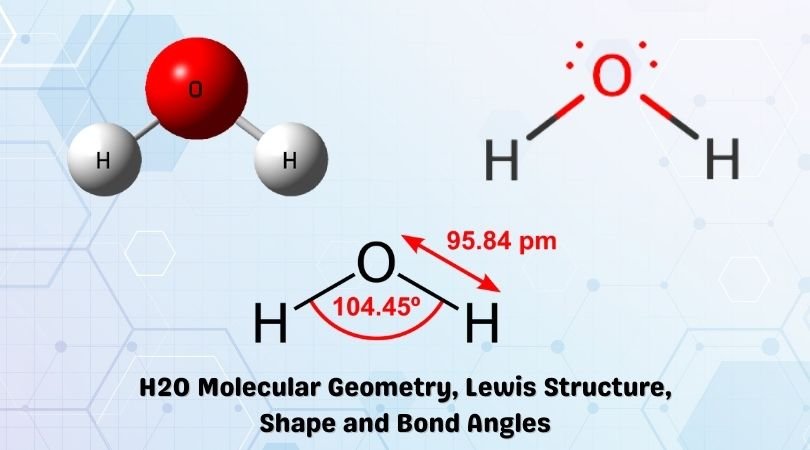
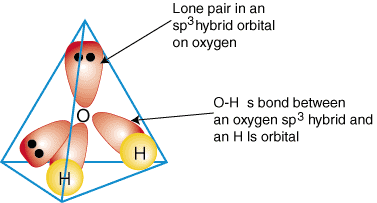
2 days ago · Molecular Orbital diagram of water (H2O) The molecular orbital diagram is a pictorial representation of determining chemical bonding between the molecules of a compound. Furthermore, the molecular orbital diagram helps with determining how two sigma bonds have been formed and the effect of the lone pairs on the structure. Figure 10.3.2 : Forming molecular orbitals for \(BeH_2\). Then we can put the Molecular Orbital diagram together, starting with the outside, drawing in bonding, non-bonding and anti-bonding MOs, and filling the electrons (Figure 10.3.3 ). The bond order is 2. Figure 10.3.3 : Molecular orbitals diagram for BeH 2. Apr 05, 2021 · Three 2p orbitals of Oxygen and one 2s orbital are hybridized as there are two pairs of bonding electrons and two lone pairs. And as four orbitals of Oxygen are hybridized, the hybridization of H 2 O is sp3. H2O Molecular Geometry. The molecular geometry of any molecule depends on its Lewis structure, the arrangement of atoms and its electrons. In polyatomic molecules we can have more than two atoms combining, e.g. in case of beryllium hydride there are 3 atoms overlapping simultaneously. So in this...
MO 4 (-6.43 eV) is the highest occupied molecular orbital (HOMO) and is essentially a (non-bonding) O-px atomic orbital. Above the HOMO is the LUMO (lowest unoccupied molecular orbital, MO 5, 1.15 eV) which doesn't contain any electrons in the ground state, as is the case for the orbitals at higher energies. MO 5 is a mixture of O-2s, O-2pz and ... Follow me on instagram-https://www.instagram.com/trickychemistrysuman/?hl=enFollow me on facebook page-https://lm.facebook.com/l.php?u=https%3A%2F%2Ffb.me%2F... Aug 29, 2018 · on H20 Molecular Orbital Diagram. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals. General procedure for simple molecules that contain a central atom: build group orbitals using the outer atoms, then interact the group orbitals. H 2 O Molecular Geometry and Bond Angles H 2 O has a tetrahedral arrangement of molecules or an angular geometry. This is mainly because the repulsion from the lone pair combination is more than bond-pair repulsion. Additionally, the existing pairs do not lie in the same plane. One pair is below the plane and the other one is above.
Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here. Chemical bonding - Molecular orbitals of H2 and He2: The procedure can ...
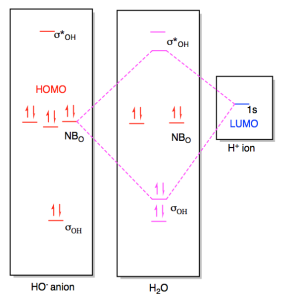
Constructing Molecular Orbital (MO) Diagram for a Non-linear Molecule like H20. Approach: Symmetry Adapted Linear Combination (SALC) Ligand Group Orbitals Steps: i. Identify and categorize the valence atomic orbitals of O using the appropriate character table.
Exercise 3.3.4. 3. Construct a qualitative molecular orbital diagram for chlorine, Cl 2. Compare the bond order to that seen in the Lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). Answer.
6.2: Molecular Orbital Theory for Larger (Polyatomic) Molecules. We can extend the method we used for diatomic molecules to draw the molecular orbitals of more complicated, polyatomic molecules (molecules with more than two atoms). To combine several different atoms in a molecular orbital diagram, we will group orbitals from different atoms ...
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
equivalent bond orbitals and sets of lone-pair orbitals. In H20, for example, there are two such sets, each with two members. In general there is an element of arbi-trariness in the definition of these sets, owing to the possibility of forming them from a linear combination of molecular orbitals of the same symmetry. Suppose, for









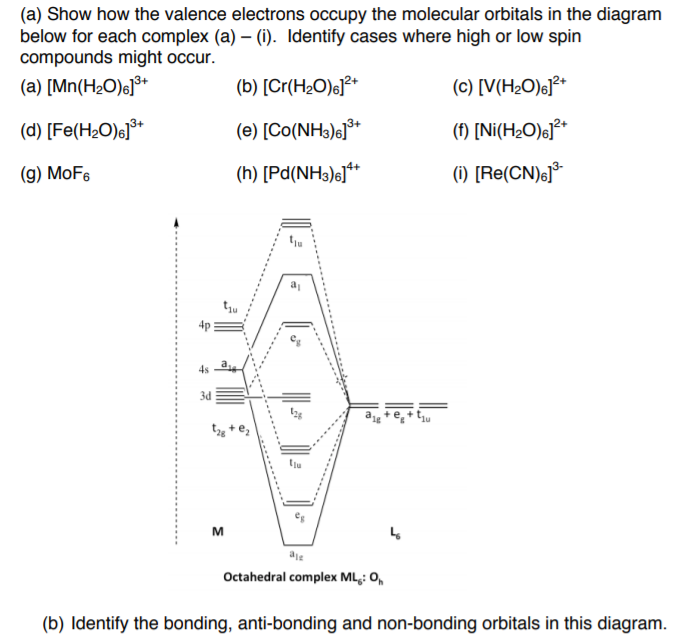
![Co(NH3)6]3+ion 4. Construct the MO diagram. Label all atomic ...](https://img.homeworklib.com/questions/54d51fd0-7560-11ea-b11c-e96f4202d848.png?x-oss-process=image/resize,w_560)











0 Response to "43 h20 molecular orbital diagram"
Post a Comment