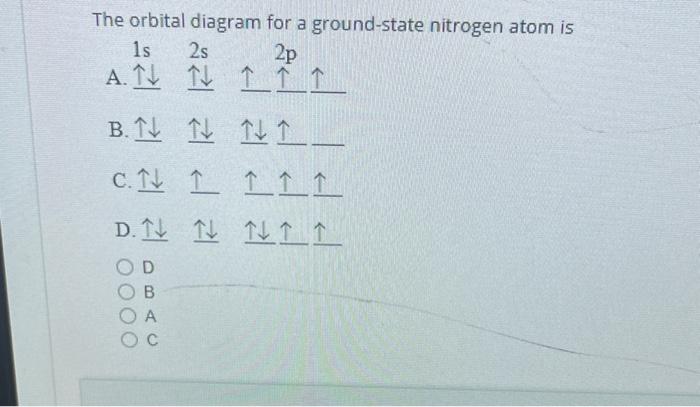
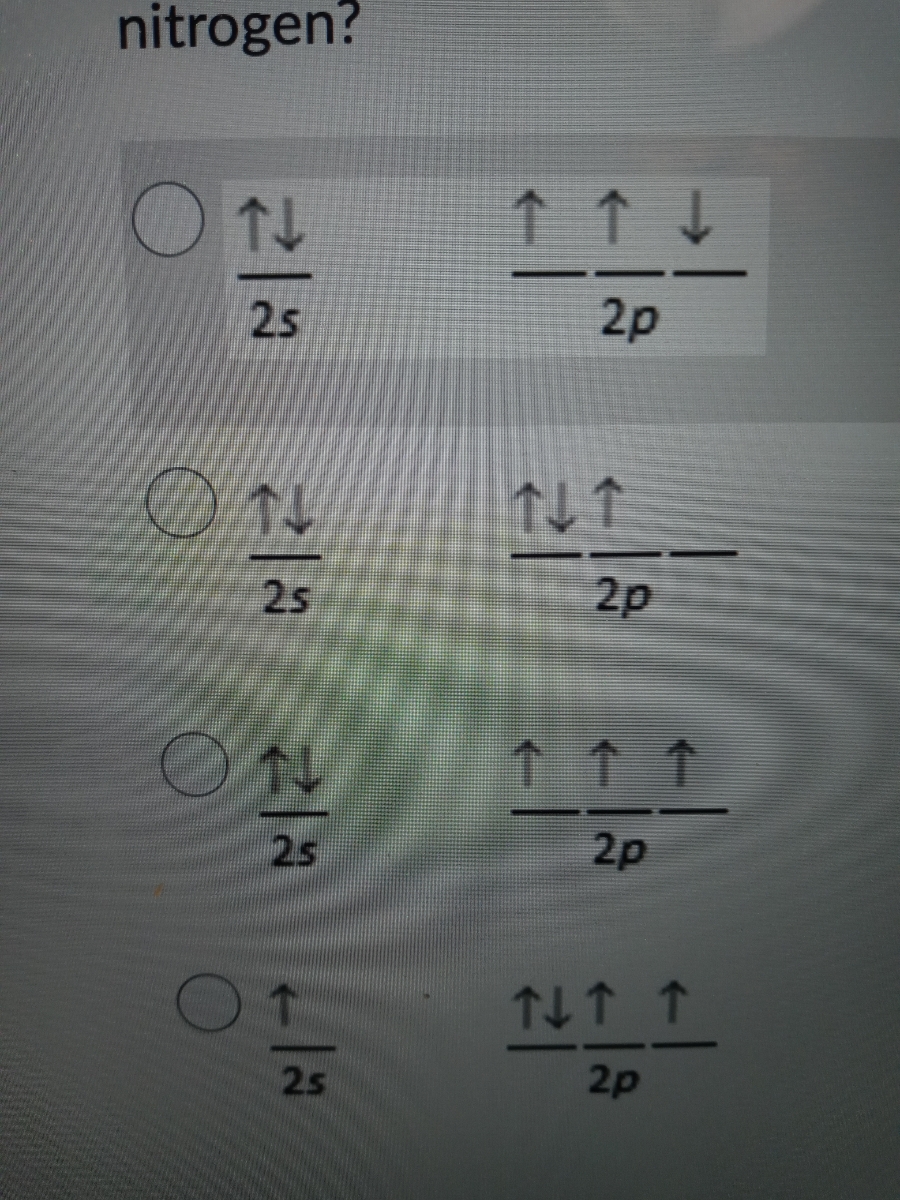
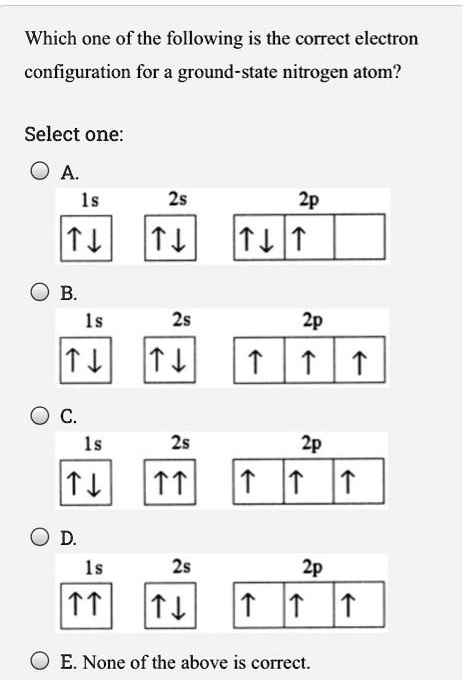
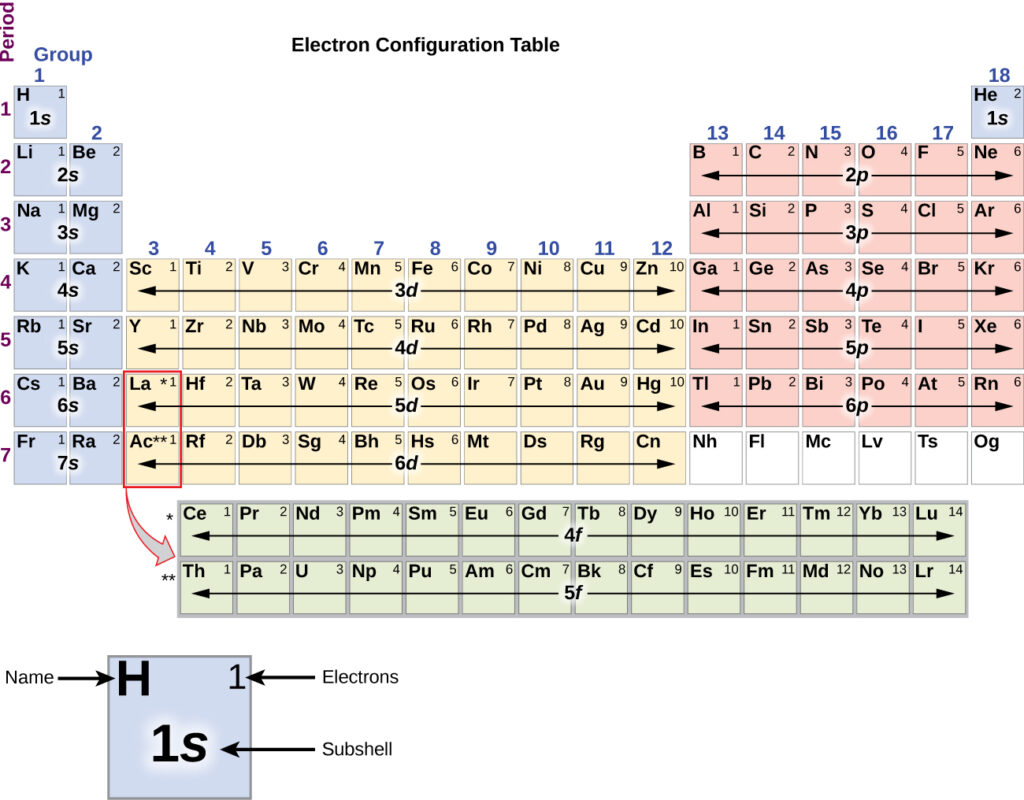
45 the orbital diagram for a ground state nitrogen atom is
SOLVED:The orbital diagram for a ground-state nitrogen atom is... In this question, we are trying to show the orbital diagram for A nitrogen atom and nitrogen is atomic # seven. Which means that it has seven Since it has seven protons and it is an atom, it will also have seven electrons. So Drawing the configure writing the configuration would give us one s... The Orbital Diagram For A Ground State Nitrogen Atom Is The 4p subshell contains three orbitals ml 1 0 1. When an electron moves from the n 1 orbit to the n 6 orbit which of the following stateme...
Answered: Here we have the molecular orbital… | bartleby 20/03/2022 · Solution for Here we have the molecular orbital energy diagram for this chlorophyll molecule. Match the absorption peaks labeled in Figure 4.7 above with the…
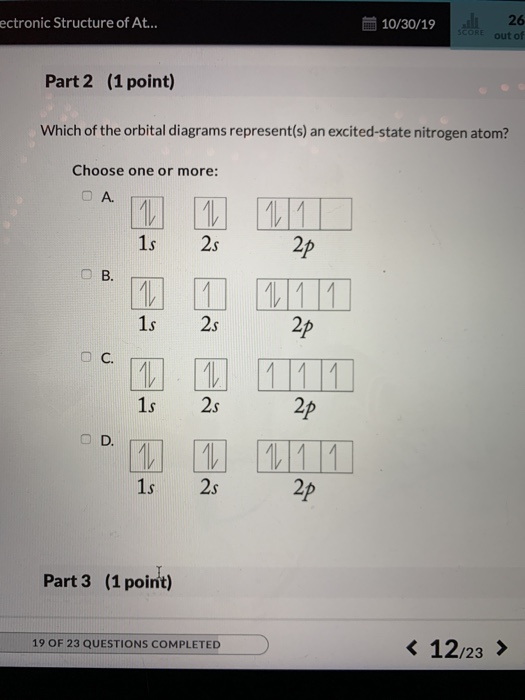
The orbital diagram for a ground state nitrogen atom is
Shapes of Orbitals | What is Orbital? Types of ... - VEDANTU The above diagram denotes the penetration decrease from s to p orbitals as the radial distribution close to the nucleus for s is more when compared to p orbitals. An ion or atom with one or more electrons occupies the higher energy orbitals and it is said to be in an excited state, whereas an ion or atom in which one or more electrons occupy low energy orbitals is said to be in its … Nitrogen - Wikipedia A nitrogen atom has seven electrons. In the ground state, they are arranged in the electron configuration 1s2 2s2 2p1 x2p1 y2p1 z. It Hypervalency is almost unknown in the 2p elements for the same reason, because the high electronegativity makes it difficult for a small nitrogen atom to be a... Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine …
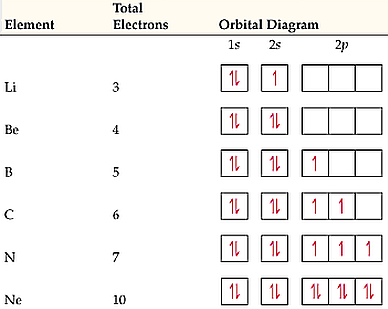
The orbital diagram for a ground state nitrogen atom is. What is the orbital diagram for a ground state carbon atom? | Socratic The ground state is 1s^2 2s^2 2p^2. In the explanation below, I show a common means of diagramming this. Using arrows to show the spin orientation of each electron, the orbital diagram is often shown this way: The single electrons in the two p-orbitals is in accordance with Hund's Rule. Oxygen(O) electron configuration and orbital diagram To create an orbital diagram of an atom, you first need to know Hund’s principle and Pauli’s exclusion principle. Hund’s principle is that electrons in different orbitals with the same energy would be positioned in such a way that they could be in the unpaired state of maximum number and the spin of the unpaired electrons will be one-way. And Pauli’s exclusion principle is that … The Orbital Diagram For A Ground State Nitrogen Atom Is Which ground state atom has an electron configuration described by the following orbital diagram. Types Of Hybridization Chemistry Assignment. By hunds rule the electron configuration of carbon which is 1s 2 2s 2 2p 2 is understood to correspond to the orbital diagram shown in c. Textbook... Nitrogen Electron Configuration (N) with Orbital Diagram Nitrogen Electron Configuration: N is one of the chemical element that has a symbol N. The atomic number of nitrogen is 7. A Scottish physician Danial Nitrogen has five valence electron in its outer shell. Ground State Electron Configuration for Nitrogen. What is the Orbital Diagram for Nitrogen?
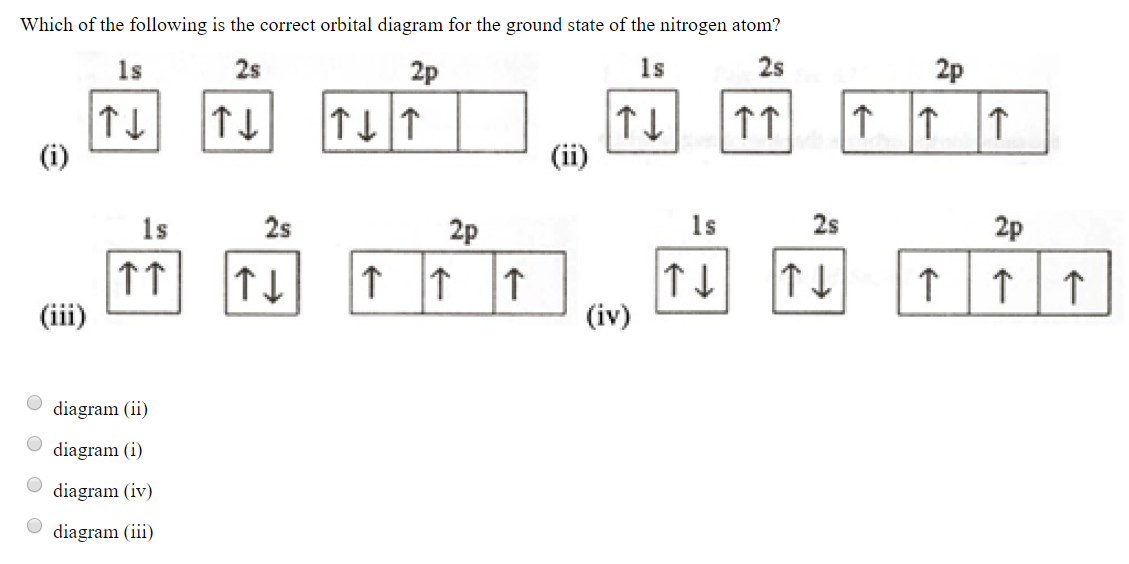
The orbital diagram for a ground state nitrogen atom... | Course Hero 23. Which ground-state atom has an electron configuration described by the following orbital diagram? (A) [Ar]4s23d9 (B) [Ar]4s13d10(C) [Ar]4s13d8 (D) [Ar]4s23d8 (E) [Ar]4s03d1027.Which of the following atoms is diamagnetic in its ground-state? Boosting oxygen reduction reaction with Fe and Se dual ... Metal(Fe) and nonmetal(Se) dual-atom sites are prepared for the first time, which displays significant enhancement for ORR. • DFT calculations indicate that the co-exist of Fe and Se dual-atomic-site is more beneficial to *OH desorption process. • Se atom has several functions: (1) adjust the electronic state of the Fe-N X. (2) Serve as ORR ... The orbital diagram for a ground-state oxygen atom... - HomeworkLib Which of the orbital diagrams represent(s) an excited-state nitrogen atom? C would be(more or less) stable than ground state C 13. Label the orbital marked above Below is the molecular orbital diagram for LiF 4 0 2s located on F 2s The diagram below is for C. 2p C 1 C2 p 0 2i C 1 2s C 2 21-0... What is electron configuration of nitrogen and copper using... - Quora Below are given the orbital diagram of Nitrogen and Copper respectively. Hope it helps! The ground state electron configuration for any atom is determined by placing the electrons in the lowest energy orbitals first, filling those before moving to the orbital with the next higher energy.
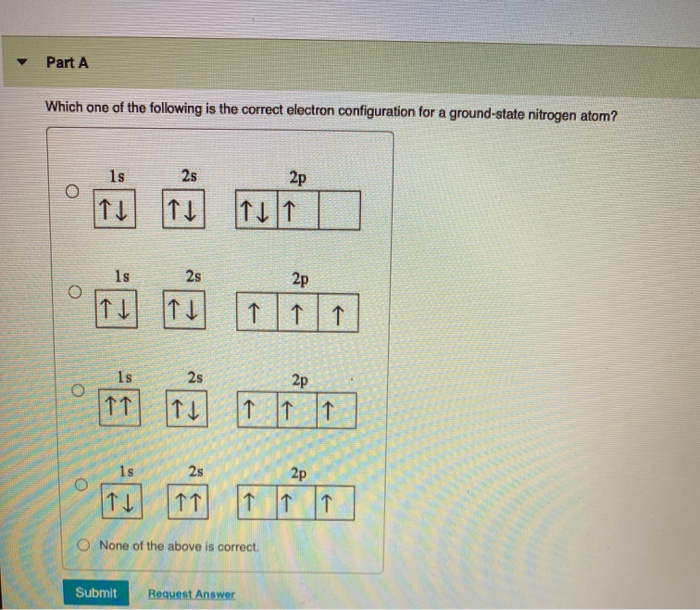
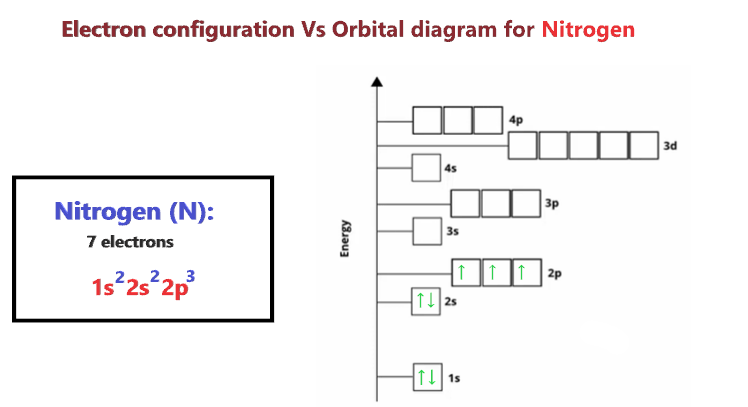
Orbital Diagram For Nitrogen (N) | Nitrogen Electron Configuration Ground State Electron Configuration For Nitrogen. When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s22s22p3. When we talk about the orbital diagram, we first need to understand what exactly it means. Quantum Numbers, Atomic Orbitals, and Electron Configurations For a hydrogen atom with n=1, the electron is in its ground state; if the electron is The distribution of electrons among the orbitals of an atom is called the electron configuration. Another way to indicate the placement of electrons is an orbital diagram, in which each orbital is represented by a square (or... Chemistry Chapter 7 Flashcards & Practice Test - Quizlet The orbital diagram for a ground-state nitrogen atom is A) A B) B C) C D) D see chart. A. The orbital diagram for a ground-state oxygen atom is A) A B) B C) C D) D E) E . D. The orbital diagram for a ground state carbon atom is A) A B) B C) C D) D. D. section 7.9 A possible set of quantum numbers for the last electron added to complete an atom of gallium (Ga) in its … Ch 7/8 quiz Flashcards | Quizlet The orbital diagram for a ground-state oxygen atom is. 1s (up down) 2s (up down) 2p (up down, up, up). Calculate the wavelength of the light emitted by a hydrogen atom during a transition of its electron from the n = 4 to the n = 1 principal energy level.
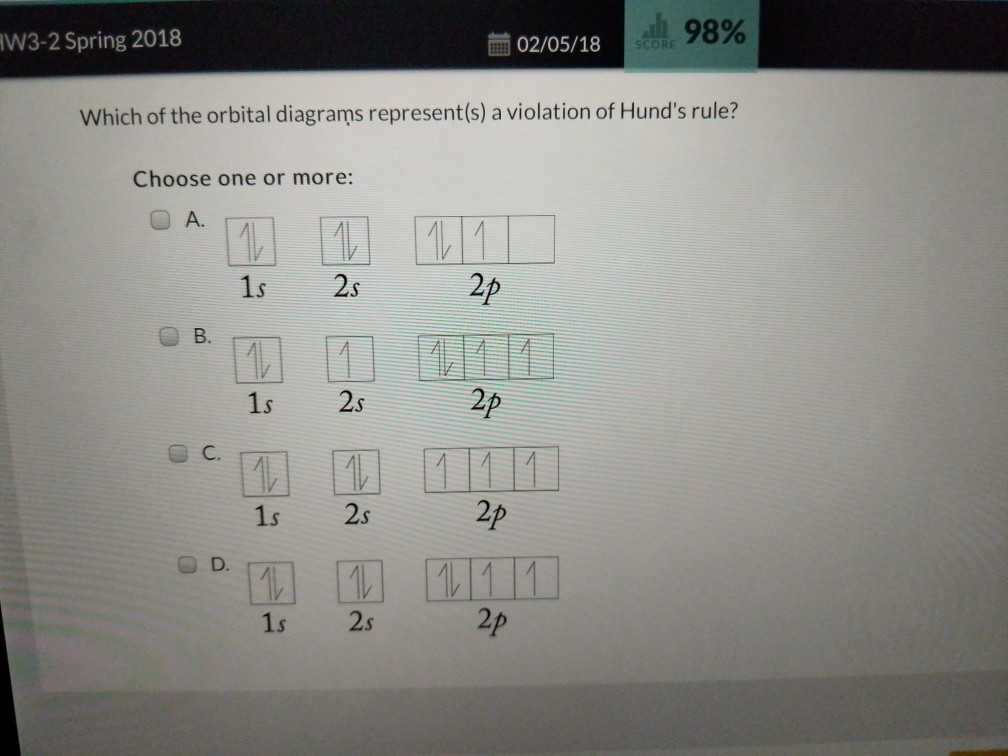
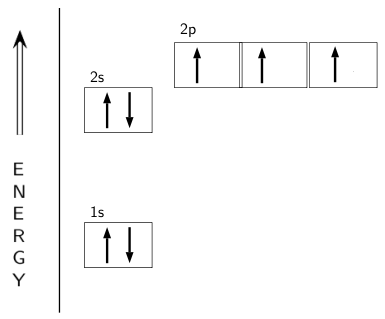
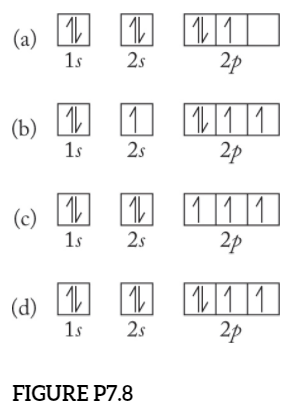
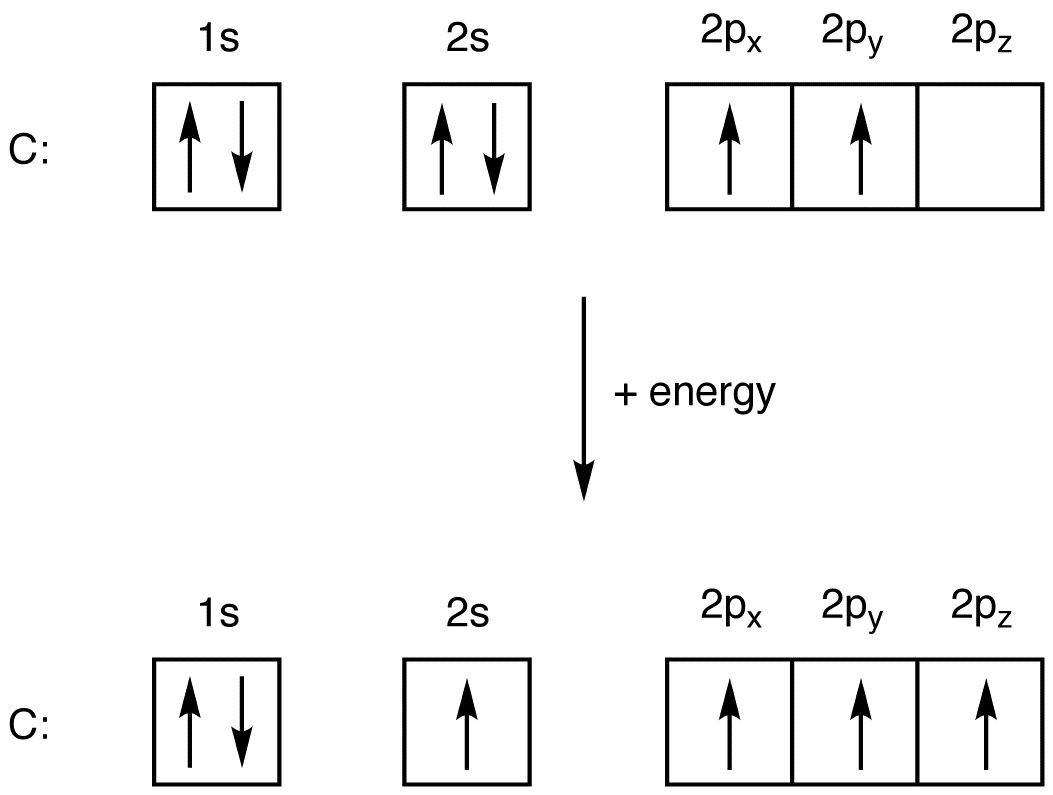
Ground state vs. excited state | Forum The ground state in an atom is when electrons are in the lowest possible energy level. The electrons in 2p were already spin paired in the orbital before each orbital contained one electron.
Elemental Nitrogen - an overview | ScienceDirect Topics The molecular orbital diagram for N2 shown in Figure 3.8 indicates that the bond order is 3 in this extremely stable molecule. The nitrogen atom has a valence shell population of 2s2 2p3 so it has a 4S ground state.
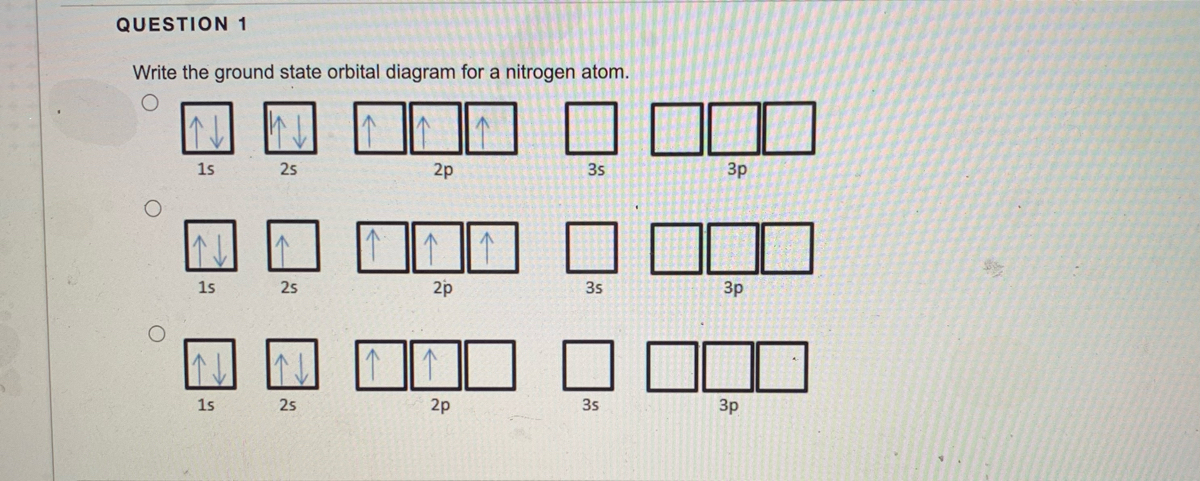
Electron Configuration - Chemistry LibreTexts 15/08/2020 · We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. The neutral atom chlorine (Z=17), for instance has 17 electrons. Therefore, its ground state electronic configuration can be …
Nitrogen(N) electron configuration and orbital diagram Table of Contents Electron configuration of nitrogen atom through orbital How to write the orbital diagram for nitrogen(N)? In the nitrogen(N) ground-state electron configuration, the three electrons of the 3p orbital are...
(Get Answer) - The Orbital Diagram For A Ground-State Nitrogen... Question 21 The Orbital Diagram For A Ground-State Nitrogen Atom Is 1s 2s 2p A. It It Î Î Î B.T Ft TuI_ C. Ît Î Î Î D. Ît Îţ Îi Î Î D A B.
Hund's Rules - Chemistry LibreTexts Atoms at ground states tend to have as many unpaired electrons as possible. The electron configuration can be written as 1s22s22p4. To draw the orbital diagram, begin with the following observations: the first two electrons will pair up in the 1s orbital; the next two electrons will pair up in...
The Orbital Diagram For A Ground State Nitrogen Atom Is What ground state atom has an electron configuration described by the following orbital. How many electrons does a fe atom have in its 3. Choose the orbital diagram that represents the ground state of n. 1b a energy is absorbed b light is emitted c the electron can have a continuous range of energies in...
The Orbital Diagram For A Ground State Nitrogen Atom Is What ground state atom has an electron configuration described by the following orbital. Answer to the orbital diagram for a ground state C has two unpaired electrons in its ground state. Nitrogen is the seventh element with a total of 7 electrons. How many electrons does a fe atom have in its 3.
Orbital hybridisation - Wikipedia In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory.For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines with three ...
Ground state electronic configuration of nitrogen atom class 11... While we were discussing the atom and its sub-shells we have studied about the charge and mass of the subatomic particles like electrons, protons and neutrons present in each atom. So now let us discuss briefly about the electronic arrangement of nitrogen atoms in the ground state.
Electron Configurations, how to write out the s p d f electronic... The list below quotes the ground state electron configurations i.e. the lowest available state Electron Box diagrams of the outer electron arrangement and examples of the simple electron notation The electrons-in-boxes notation for subshells: Boxes are used to represent an individual orbital or set of...
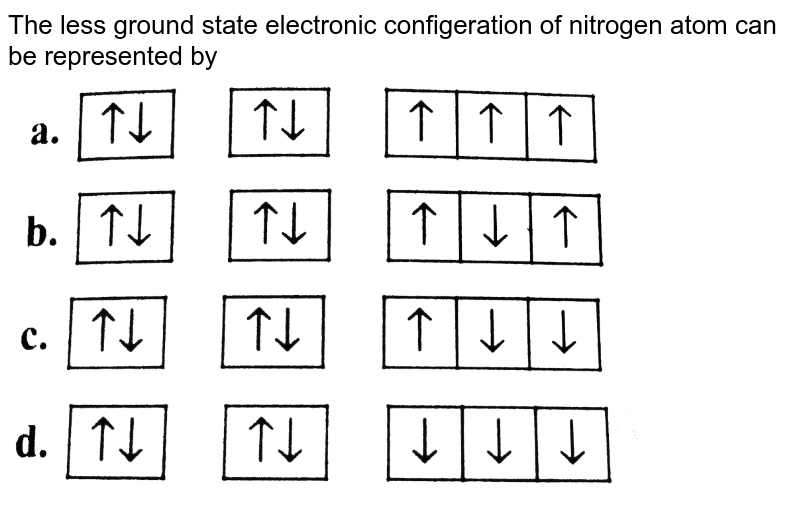
[SOLVED] Ground state configuration of nitrogen atom can be > The orbital diagram in which both Pauli's exclusion principle and Hund's rule violated is Atoms Chemical Kinetics Moving Charges and Magnetism Microbes in Human Welfare Semiconductor Electronics: Materials, Devices and Simple Circuits.
Orbital Filling Diagram For Nitrogen - Drivenhelios Show the orbital filling diagram for rm n nitrogen best answer the electronic configuration for nitrogen atom is 1s 2 2s 2 2p 3 lowest energy state Solved Show The Orbital Filling Diagram For S Sulfur S. What Is The Configuration Of Sulphur Quora. Qualitative Valence Molecular Orbital Mo...
Valence Bond Theory | Boundless Chemistry - Lumen Learning Boron configuration diagram: One of the three boron electrons is unpaired in its ground state. The atomic s- and p-orbitals in boron’s outer shell mix to form three equivalent hybrid orbitals. These particular orbitals are called sp 2 hybrids, meaning that this set of orbitals derives from one s- orbital and two p-orbitals of the free atom.
MCQ Questions for Class 11 Chemistry Chapter ... - Learn Cram 17/06/2021 · If the nitrogen atom had electronic configuration 1s², it would have energy lower than that of the normal ground state configuration 1s² 2s² 2p³, because the electrons would be closer to the nucleus. Yet, 1s² is not observed because it isolates. (a) Heisenberg’s Uncertainty Principle (b) Hund’s rule (c) Pauli Exclusion Principle (d) Bohr postulate of stationary orbits. …
WebElements Periodic Table » Nitrogen » properties of free atoms The ground state electron configuration of ground state gaseous neutral nitrogen is [He].2s2.2p3 and the term symbol is 4S3/2. Schematic electronic configuration of nitrogen. The Kossel shell structure of nitrogen. Atomic spectrum.
PDF Microsoft Word - Chapter Five | Structure of Atoms Key The orbital diagram for a ground-state oxygen atom is. A. phosphorus B. nitrogen C. arsenic D. vanadium E. none of these. 39. How many unpaired electrons does a ground-state atom of sulfur have?
Chem I Review Part 2 The orbital diagram for a ground-state oxygen atom is A. Row 1. B. Row 2. C. Row 3. D. Row 4. E. Row 5. 18. Which ground-state atom has an electron configuration described by the following orbital diagram? A. phosphorus B. nitrogen C. arsenic D. vanadium E. none of these 19.
Solved The orbital diagram for a ground-state nitrogen atom Transcribed image text : The orbital diagram for a ground-state nitrogen atom is 1s 25 2p A A 신 소 소 소 B.소 솨 솨 소 C C. 소 소 소 소 소 소 D. 신 신 소 소.
Orbital Diagrams - Concept - Chemistry Video by Brightstorm Alright let's talk about orbital diagrams. Orbital diagrams are a pictorial description of electrons in an atom. In order to figure out where electrons go in an Let's put all these stuff into play, how this all come together. Okay let's do the orbital diagram for iron, iron we know is on its ground state of 26...
en.wikipedia.org › wiki › Glossary_of_chemistry_termsGlossary of chemistry terms - Wikipedia Also acid ionization constant or acidity constant. A quantitative measure of the strength of an acid in solution expressed as an equilibrium constant for a chemical dissociation reaction in the context of acid-base reactions. It is often given as its base-10 cologarithm, pK a. acid–base extraction A compound which, when dissolved in water, gives a pH of less than 7.0, or donates a hydrogen ...
What is the orbital diagram for a ground-state nitrogen atom? What orbital diagram correctly represents the outermost principal energy level of a nitrogen atom in the ground state?
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine …
Nitrogen - Wikipedia A nitrogen atom has seven electrons. In the ground state, they are arranged in the electron configuration 1s2 2s2 2p1 x2p1 y2p1 z. It Hypervalency is almost unknown in the 2p elements for the same reason, because the high electronegativity makes it difficult for a small nitrogen atom to be a...
Shapes of Orbitals | What is Orbital? Types of ... - VEDANTU The above diagram denotes the penetration decrease from s to p orbitals as the radial distribution close to the nucleus for s is more when compared to p orbitals. An ion or atom with one or more electrons occupies the higher energy orbitals and it is said to be in an excited state, whereas an ion or atom in which one or more electrons occupy low energy orbitals is said to be in its …




















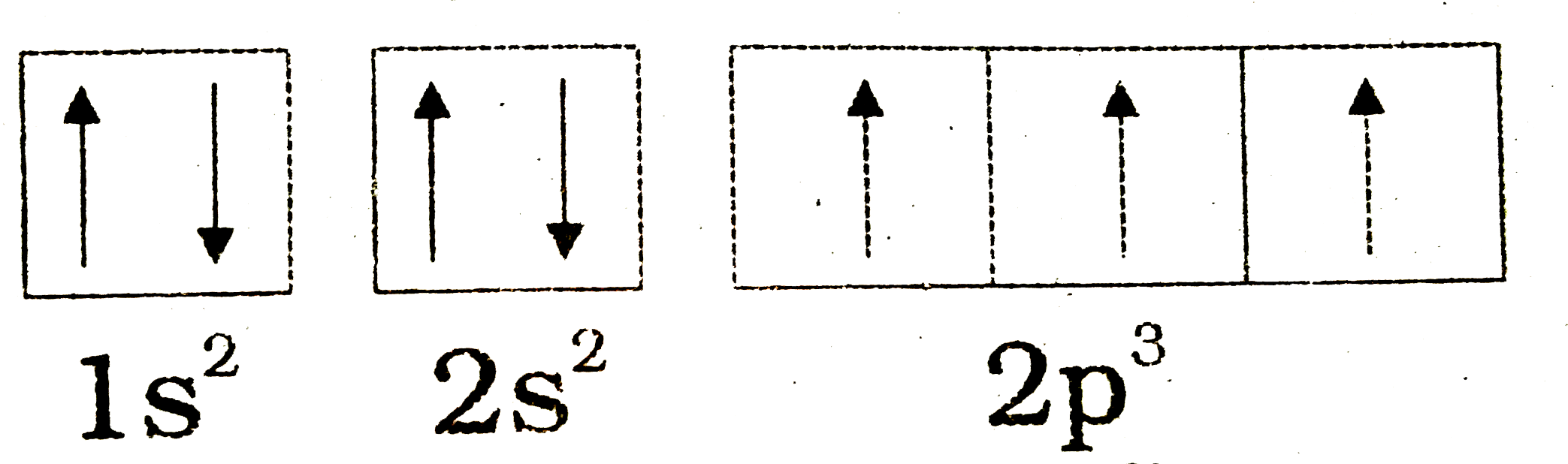





0 Response to "45 the orbital diagram for a ground state nitrogen atom is"
Post a Comment